Summary
A cubital vein is one of the most frequently and widely utilized vessels for intravenous drug delivery and blood sampling in clinical practice. However, no studies thus far examined the feasibility and safety of catheter-directed thrombolysis (CDT) by using a single superficial cubital vein access in patients presenting with acute pulmonary embolism (PE). This study showed that the pharmacoinvasive approach by using CDT and local alteplase delivery was associated with significant hemodynamic improvement in patients with acute PE, while not causing any life-threatening bleeding, access-related bleeding, or major periprocedural complication. Furthermore, CDT through the cubital vein for treatment of acute PE appears to be a technically simple, cost-effective, and safe treatment approach while its use should be systematically examined in future large prospective studies, especially in comparison to traditional femoral or jugular venous approaches and in scenarios of high-risk PE in which patients have an indication for systemic thrombolysis but might be at high risk of major bleeding.
Introduction
Pulmonary embolism (PE) is one of the most common life-threatening emergencies in which survival greatly depends on hemodynamic stability. The rate of in-hospital mortality in hemodynamically unstable patients with PE is high and reported to reach nearly 32% [1]. Treatment options for PE include systemic thrombolysis, catheter-directed interventions, and surgical thromboembolectomy. Catheter-directed therapy (CDT) entails mechanical fragmentation of the clot, removal of obstructing thrombi from the main to distal pulmonary arteries, and thrombolytic-enhanced clot lysis. This intervention provides a rapid reduction of pulmonary artery pressure, right ventricle (RV) strain, and pulmonary vascular resistance while simultaneously increasing systemic perfusion and facilitating RV recovery [2].
Despite guideline recommendations, about two-thirds of patients with massive PE and hemodynamic instability do not receive thrombolytic therapy whereas this trend is even more pronounced among patients with submassive PE [3]. The risk of major bleeding is an important factor implicated in the underuse of systemic thrombolysis [4]. For example, full-dose systemic thrombolysis (100 mg of alteplase over 2 h) carries up to a 20% risk of major bleeding, including a 2% to 5% risk of intracranial hemorrhage [5]. In such circumstances, CDT might be an attractive treatment option offering local application of thrombolytics thus significantly reducing the risk of major bleeding. In recent CDT trials, the thrombolytic dose was significantly reduced (10 to 28 mg of alteplase over 12–24 h) and no intracranial hemorrhage in patients with acute PE was observed [6–8].
Regarding the vascular access in CDT, proximal venous sites are most commonly used such as femoral or jugular [6–10]. Mechanical complications such as perforation or dissection of major pulmonary artery branches, severe pulmonary hemorrhage, or pericardial tamponade were sporadically reported [11]. However, the majority of periprocedural complications were access-related such as hematoma at the access site, the formation of arteriovenous fistula or pseudoaneurysm due to femoral or carotid artery puncture, or pneumothorax [7, 8, 12, 13]. Recently, Roule and colleagues demonstrated that cubital venous access for right heart catheterization was feasible in the large majority of patients, with a low incidence of periprocedural complications, shorter fluoroscopy time, three-times lower radiation dose, and less access-site hematomas compared to the femoral approach with the recommendation that cubital access should have preferential use in experienced centers [14].
To our best knowledge, this is the first literature report on CDT via superficial cubital vein access in the setting of submassive and massive PE that examined feasibility and vascular and systemic complications of such approach. Thus far this approach has only been sporadically reported in a case series.
Aim
The purpose of the present study was to investigate the feasibility and safety of superficial cubital venous access for the provision of CDT in the treatment of acute PE.
Material and methods
Patient recruitment and blood sampling
Patients were consecutively and prospectively enrolled in the study during the period from January 2016 to March 2019 if they met all the following inclusion criteria: age ≥ 18 years and objectively confirmed acute massive or submassive PE with an onset of symptoms 14 days or less before enrollment. Diagnosis of PE had to be confirmed by computed tomographic (CT) angiography or pulmonary angiography. Patients were excluded from participation if they had any absolute contraindication to thrombolytic therapy defined by 2019 European Society of Cardiology (ESC) guidelines on the diagnosis and management of acute PE: hemorrhagic stroke or stroke of unknown origin at any time, ischemic stroke in the preceding 6 months, central nervous system damage or neoplasms, recent major trauma/surgery/head injury in the preceding 3 weeks, active bleeding or bleeding diathesis [15]. All patients who met inclusion criteria gave consent to participate in the study or they agreed that their anonymized data can be used for the study purposes. This consent was prospectively obtained in written form from patients that were fully conscious and hemodynamically stable while those with severe hemodynamic instability agreed that their data can be used for scientific purposes after they were clinically stabilized with the aforementioned procedure. This study is approved by the institutional Ethics Committee and filed under number 500-03/21-01/178.
PE type was classified based on the definition provided by the American Heart Association (AHA) – acute massive or high-risk PE was defined as an acute PE with systemic hypotension, a requirement for vasoactive drugs, or a need for cardiopulmonary resuscitation. Submassive or intermediate-risk PE was defined as the absence of hypotension but the presence of the right ventricle (RV) dysfunction determined by CT, echocardiography, or biomarkers of cardiac necrosis. Furthermore, criteria for right heart dysfunction included an elevated RV/LV ratio ≥ 0.9 [1]. Additional risk stratification was undertaken according to the European Society of Cardiology (ESC) guidelines for the diagnosis and management of acute PE [15]. Patients that were hemodynamically unstable meaning that they had resuscitated cardiac arrest, obstructive shock, or persistent hypotension were classified as high-risk PE patients. Similarly, patients with an absence of hemodynamic instability but with an sPESI score ≥ 1, signs of RV dysfunction shown on transthoracic echocardiography or CT pulmonary angiography and elevated cardiac troponin levels were designated as an intermediate-high risk. Since all of our intermediate-risk patients had signs of RV dysfunction on imaging, those without elevated cardiac troponins were designated as intermediate-low risk, as per ESC guidelines. Age, height, weight, and body mass index (BMI), length of hospitalization, as well as levels of hemoglobin, D-dimers, high-sensitivity cardiac troponin I (hs-cTnI), N-terminal pro-brain natriuretic peptide (NT-proBNP), C-reactive protein (CRP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), ᵞ-glutamyltransferase (GGT), and lactate dehydrogenase (LDH) were determined before the CDT procedure. Systolic and diastolic arterial blood pressure at baseline and post-CDT were measured using a mercury manometer. All patients were monitored by the standard limb leads electrocardiogram during the procedure and this was also used to determine baseline and post-CDT heart rate (HR). Systolic and mean pulmonary artery pressures were determined over a pigtail catheter positioned in the pulmonary artery before and after the procedure. The Miller score was calculated in each patient to grade the severity of PE as judged by the angiographic findings of obstruction and perfusion, before and after the treatment, ranging from 0 to 34 points [16]. The Miller Index is derived by dividing the Miller score by 34 (range 0 to 1). Shock index (SI) as a measure of shock severity was calculated as the heart rate in beats per minute divided by the systolic arterial blood pressure in mm Hg [17]. A simplified Pulmonary Embolism Severity Index (sPESI) score was calculated and patients having sPESI score ≥ 1 were classified at the high risk of death at 30 days [18].
Procedure and techniques used for the pharmacoinvasive approach
All procedures were performed in the cardiac catheterization laboratory by an experienced interventional cardiologist with high procedural volumes. The median cubital venous puncture, mostly on the right arm, was performed by experienced nurses trained for this procedure. Briefly, a tourniquet was applied to the upper arm, away from the disinfected area, and the cubital vein was identified by palpation. The vein was punctured using an 18- or 20-gauge intravenous catheter. The tourniquet was moved and the area around the intravenous catheter was re-disinfected using antiseptic detergent and alcoholic disinfectant (PLIVA®sept foaming, Zagreb, Croatia). After administering a local anesthetic, a 0.038-inch wire from the introducer set was passed through an intravenous catheter, and an intravenous catheter was pulled out. Over the wire, a 5F introducer sheath (Radiofocus® Introducer II, Terumo Europe NV, Leuven, Belgium) was placed. Using a 0.035-inch hydrophilic guidewire (Radiofocus® M, Terumo Europe NV, Leuven, Belgium) the 5F Multipurpose (MP) or JR angiographic catheter (Multipurpose, Terumo Europe NV, Leuven, Belgium; or JR 4, Cordis Corporation, Florida, USA) was advanced under fluoroscopic guidance and was placed into the pulmonary artery. The hydrophilic guidewire was then replaced with 300 cm long 0.035-inch J-tip guide wire (EmeraldTM, Cordis, Cashel, Co Tipperary, Ireland). Then over a standard wire, a 5F angiographic catheter was replaced with a 5F pigtail catheter (Infiniti® PIG 155°, Cordis Corporation, Florida, USA) and pulmonary artery pressure was measured. Thereafter a pigtail catheter was used to obtain pulmonary angiography with an injected volume of 30 ml of a non-ionic contrast agent (OmnipaqueTM 350, GE Healthcare, Pittsburgh, PA, USA) by using a pump injector (flow of 15 ml/s) first in one of the main branches (right or left) of the pulmonary artery (Figures 1 A, B). Subsequently, after obtaining pulmonary angiography a mechanical fragmentation of the embolus was performed by back-and-forth movement and rotation of pigtail catheter and partly using back and forth movements of standard J-tip wire. After mechanical fragmentation, an initial bolus dose of 2.5 mg of tissue plasminogen activator (t-PA) alteplase (Actilyse, Boehringer Ingelheim, Ingelheim, Germany) was given. The catheter was then relocated to another main branch of the pulmonary artery and the procedure was repeated. The pigtail was left in the proximal embolus of the more affected branch for next 12 h for subsequent local thrombolytic therapy with continuous infusion of 25 mg of alteplase. Therefore, our protocol utilized a total of 30 mg of alteplase per patient. The cubital region and an external part of the introducer sheet and pigtail catheter were protected for the total duration of the whole procedure and during infusion protocol with a transparent film dressing (3MTM TegadermTM) that provided a waterproof and sterile barrier to external contaminants including liquids, bacteria, and viruses.
Figure 1
A, B – Pulmonary angiogram of pulmonary arteries prior to catheter-directed thrombolysis (CDT), C, D – 12 h after CDT showing the almost complete resolution of the thrombus and recanalization of the pulmonary arterial blood flow
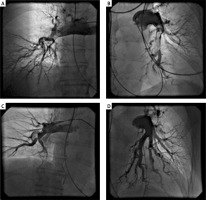
During the continuous thrombolytic infusion, hemodynamic and electrocardiographic monitoring was performed in the Intensive Coronary Care Unit within the Cardiology Department. After 12 h of continuous infusion of the local thrombolysis, post-CDT pulmonary angiography (Figures 1 C, D) and pulmonary artery pressure measurement were performed again. After the procedure, the pigtail catheter was carefully extracted from the pulmonary artery (always over the guidewire to avoid damage to the pulmonary valve, tricuspid valve, and/or sensitive vein structures). Closure of the venous access was managed by manual compression for up to a few minutes, followed by a mild compressive bandage for 2 h. Simultaneously with t-PA, patients received the full-dose intravenous unfractionated heparin (UFH) with a target activated partial thromboplastin time (aPTT) of 50 to 75 s. After UFH and the t-PA application were completed, low molecular weight heparin (LMWH) adjusted to body weight in two daily doses was administered over the next 24 h. Finally, LMWH was switched to peroral anticoagulant therapy. Finally, all patients enrolled in the study were discharged home with the prescription of anticoagulant therapy (warfarin or novel oral anticoagulant) for at least 6 months.
Statistical analysis
Data were expressed as mean (standard deviation, SD). The normality of the distribution was confirmed for all parameters by using the Shapiro-Wilk test. All of the comparisons of parameters measured pre-CDT versus post-CDT were performed by Student’s t-test for comparison of dependent samples. Analyses were done with SPSS Statistics for Windows® (version 25.0, IBM, Armonk, NY, USA) and Prism 6 for Windows® (version 6.01, GraphPad, La Jolla, CA, USA). All p-values were two-tailed and considered significant if < 0.05.
Results
Ten out of 27 (37%) patients fulfilled the criteria for massive PE, others had submassive PE. According to ESC 2019 classification for PE, 10 (37%) patients were classified as high risk, 11 (41%) were of intermediate-high risk while 6 (22%) were at intermediate-low risk while no patients with low risk were enrolled. A total of 27 (13 males) patients with an average age of 60.6 (14.1) years participated in the study. The mean BMI was 30.3 (4.5) kg/m2. The baseline patient characteristics are summarized in Table I. Regarding the biochemical parameters, mean LDH levels were slightly above the upper normal range. Hepatic and cholestatic parameters including AST, ALT, and GGT levels were ~1.5 times higher with respect to the upper normal range, while levels of D-dimers, hs-Troponin I, NT-proBNP, and CRP were significantly elevated above the upper normal range (~10 to 20 times higher).
Table I
Anthropometric and laboratory data on admission (pre-CDT application)
[i] Data are presented as mean (SD). BMI – body mass index, CDT – catheter-directed thrombolysis, NT-proBNP – N-terminal pro-brain natriuretic peptide, hs-cTnI – high-sensitivity cardiac troponin I, CRP-C – reactive protein, AST – aspartate aminotransferase, ALT – alanine aminotransferase, GGT – ᵞ-glutamyltransferase, LDH – lactate dehydrogenase.
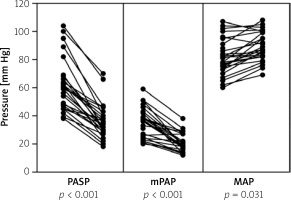
The procedural success concerning right heart catheterization via cubital vein was achieved in all 27 patients. In all patients, significant improvement in hemodynamics and pulmonary angiography was observed 12 h after the procedure (Table II). As shown in Figure 2, invasively measured hemodynamic parameters including systolic and mean pulmonary artery pressures decreased from 61.4 (18.3) mm Hg and 35.7 (10.8) mm Hg at baseline to 35.8 (12.3) and 21.1 (6.5) mm Hg at the completion of the procedure (mean difference, –25.6 mm Hg and –14.6 mm Hg; p < 0.001, respectively). Concordantly, mean arterial pressure as a reflection of systemic perfusion pressure significantly improved (from 81.9 (12.8) to 89.0 (10.3) mmHg (p = 0.031, Figure 2). The heart rate also significantly decreased from baseline to post-procedure (103.9 (17.6) vs. 78.0 (12.5) bpm; p < 0.001). Furthermore, the Shock Index as a bedside assessment of circulatory shock significantly improved from baseline to post-CDT (1.01 (0.28) vs. 0.66 (0.18), p < 0.001). Pretreatment Miller angiographic obstruction score and index decreased from 25.7 (3.6) and 0.75 (0.11) to 11.8 (4.0) and 0.34 (0.12) post-CDT (p < 0.001, respectively). The average length of hospitalization was 6 ±3 days.
Table II
Hemodynamic and angiographic data before and after the CDT procedure
Figure 2
Before-after graph showing pre-CDT versus post-CDT values of pulmonary artery systolic pressure (PASP), mean pulmonary artery pressure (mPAP), and mean arterial pressure (MAP) among all enrolled patients
The rates of adverse events were low. Bleeding events occurred in 2 out of 27 patients (7.4%). One patient with massive PE and chronic lymphocytic leukemia had profuse epistaxis that required anterior nasal packing and transfusion of 1 unit of packed red blood cells. In another patient with submassive PE, thrombolytic therapy was terminated 2 h after starting due to the development of subcutaneous hematoma at the site of the former traumatic injury experienced during syncope related to acute PE onset (data not included in statistical analysis) which did not require transfusion of blood products. Intracranial hemorrhage as well as access-site hematoma or access-related complications were not observed. During the procedure in one female subject paroxysm of atrial fibrillation was recorded. However, after intravenous administration of 300 mg of amiodarone, the patient converted to the sinus rhythm within few minutes. There were no other periprocedural complications or serious adverse effects related to the study treatment. A descriptive comparison of safety and efficacy outcomes in this study with studies using other access points is provided in Table III.
Table III
Comparison of safety and efficacy outcomes between current study utilizing superficial transcubital venous access and notable studies using jugular and/or femoral access for catheter-directed thrombolysis in acute pulmonary embolism
| Variable | Jugular access | Femoral access | Cubital access |
|---|---|---|---|
| ULTIMA study [27] Femoral vein N = 30 pts | 100% placement success No major bleeding 3 minor bleeding events (10%) | ||
| SEATTLE II study [28] Common femoral vein in 85.6% of cases N = 150 pts | 97.5% placement success 16 major bleeding events within 72 h of procedure (10.7%) 14 GUSTO moderate bleeding events (9.3%) | ||
| Pelliccia et al. [29] Femoral vein Percutaneous rheolytic thrombectomy N = 33 pts | 97% procedural success No major bleeding events 9 transient periprocedural side effects (27.2%) 4 (12.1%) cases of anemia | ||
| PERFECT study [30] Jugular or femoral access N = 101 pts | Transjugular or transfemoral access: 85.7% clinical success in massive PE 97.3% clinical success in submassive PE 12.9% of minor bleeding events No major procedure-related complications, no major bleeding and no hemorrhagic strokes | ||
| Present study Superficial cubital vein N = 27 | 100% clinical success 2 (7.4%) bleeding events that required physician intervention No procedure-related complications, no major bleeding or hemorrhagic strokes | ||
Discussion
This is the first-in-literature analysis of superficial cubital venous access for acute PE treatment, provided in more than a case series presentation, thus including analysis of vascular and systemic complications of such treatment. Although cubital vein access has been previously examined and documented for a right heart catheterization in different clinical scenarios, it has not been tested as an interventional approach in the setting of acute PE.
In this study, we demonstrated that this treatment option was safe, technically feasible, and associated with significant hemodynamic improvement among patients with PE.
The objective of the invasive approach is the degradation and dissolution of obstructing thrombi from the main pulmonary arteries to unload RV strain and to rapidly improve pulmonary perfusion [2]. Such effects are accomplished by catheter-directed fragmentation that facilitates recanalization of central embolic occlusion. Of note, fragmentation of central emboli and dislocation of the fragments to the periphery results in a relative gain of non-obstructed cross-sectional area in patients threatened by RV failure in whom even a small hemodynamic improvement may be lifesaving [10]. Moreover, the increased total surface area of the fragments may accelerate the efficacy of an accompanying thrombolytic or spontaneous intrinsic fibrinolytic activity [10]. In comparison with the full-dose systemic intravenous administration of thrombolytics, the local application provides additional substantial benefits. Particularly, CDT enables the reduction of the thrombolytic dose with a similar effect on the clot lysis, while significantly reducing the risk of bleeding [18]. On the other hand, contrary to invasive open surgical thrombectomy, which has been associated with high perioperative morbidity and mortality, percutaneous CDT represents a safer and less invasive option for treating patients with acute PE [12]. Furthermore, a catheter positioned in the pulmonary artery enables continuous assessment of pulmonary hemodynamics, follow-up angiography, and additional intervention/s if needed.
Thrombolysis as the gold standard therapy in acute massive PE (high-risk PE) should be considered for the majority, if not all the patients. However, thrombolysis is used infrequently despite the potential clinical benefit of rapid clot lysis, mainly due to the inherent risk of significant bleeding complications. A study by Patel et al. revealed alarming data – out of 110,731 patients hospitalized with PE, only 1,521 (1.37%) were treated with thrombolysis. Of those treated with thrombolysis, 76.8% received systemic intravenous thrombolysis while 23.2% received CDT thus reflecting that the clinical fear concerning thrombolysis use in PE is substantial [19]. Regardless, thrombolysis remains to be a double-edged sword in acute PE where intracranial and even fatal hemorrhage are well-known complications [13, 20, 21].
The ideal approach would be to find a thrombolytic dose that would keep the maximum effect on the clot with a minimal bleeding risk. Recently, three studies on CDT for acute PE (ULTIMA, PERFECT, and SEATLE II) with similar t-PA doses used as in the presented study (total 10 or 20 mg, 28 mg, and 24 mg; respectively; in our study total of 30 mg of alteplase) have reported total bleeding events at a rate of 10%, 12.9%, and 11.3%. Of the total number of bleeding events 33% in ULTIMA, 54% in PERFECT, and 24% in the SEATLE II study were related to the access site (femoral or jugular), while in our study no access-site (cubital) bleeding events were observed [6–8]. Of note, the average procedural success rate in the PERFECT study that used various infusion catheters was 94%, SEATTLE II study using EKOS endovascular system reported a placement success of 97.5% while a complete procedural success with using EKOS device in the ULTIMA trial was achieved. A very recent study conducted in 33 patients with massive PE by using the AngioJet catheter reported an angiographic success in 96% of patients with no major bleeding events, distal embolizations, or perforations of pulmonary arteries while transient periprocedural side effects such as heart block, hypotension, and bradycardia were reported in 1, 3, and 5 patients, respectively [22]. However, it should be noted that these systems were placed by using large-bore access through magistral veins, most of them used echocardiographic guidance to make successful insertion and in some instances, these systems were placed bilaterally thus enhancing periprocedural risks.
On the other hand, in our study, we did not use ultrasound to perform puncture to the cubital vein and we used a standard workhorse 5F pigtail catheter that is likely more readily available in the cath lab compared to other dedicated systems. Similarly, we did not use the ultrasound-accelerated thrombolysis or the aspiration of the central clot either by manual aspiration/suction embolectomy, AngioJet/rheolytic thrombectomy, and/or rotational thrombectomy and once the catheter is firmly positioned in the pulmonary artery, all of these adjuvant methods are available for potential use.
Ultrasound-assisted thrombolysis might also be utilized instead of standard CDT, although it was shown in the Standard vs. Ultrasound-Assisted Catheter Thrombolysis for Submassive Pulmonary Embolism (SUNSET sPE) trial that such approach achieved a similar pulmonary arterial thrombus reduction compared to standard CDT in patients with submassive PE [23]. Outcomes in intermediate-high and high-risk PE can also be improved by organizing PE response teams (PERT) capable of providing CDT to patients with contraindications to fibrinolysis or those after failed fibrinolysis while such organizational pathway proved to be useful in special circumstances such as COVID-19 pandemic [24, 25].
The cubital venous approach, as once transradial in left heart catheterization offers an interesting alternative to the proximal venous approaches in catheter-directed pharmacomechanical treatment of acute PE. It was recently shown in a large study that catheterization duration, fluoroscopy time, and consequently radiation dose were significantly reduced in patients undergoing right heart catheterization through antecubital venous access in comparison to femoral access, although this study was not focused on PE [14]. A similar favorable profile was confirmed in a study focused on the hybrid use of the antecubital fossa vein and radial artery for bilateral catheterization of patients with heart failure [26]. The approach reported herein provides relatively easier navigation (single S angulation) through the right heart chambers when approached from a superior rather than an inferior vena cava, in which navigation often faces a double S angulation. Indeed, right heart catheterization from the superior vena cava follows a natural winding course, a simple curve. In comparison with the proximal approaches, cubital venous access carries a lower risk of access-site bleeding and access-related complications. Cannulation of the cubital vein, especially among experienced operators, usually does not require multiple punctures that increase the risk of local complications. Even more, there are no major arteries in the vicinity of the cubital vein that might be susceptible to iatrogenic injury. A cubital vein, in comparison to a femoral or jugular vein, is easier to compress and control in the case of local complications. In the end, we assume that the managing procedure through a cubital vein in comparison to proximal vein accesses (especially transjugular) would be more acceptable to patients, causing less anxiety.
This study has some limitations. Of note, a relatively low patient sample was enrolled, therefore, with a larger number of patients it is possible that procedural complications would be higher and success rates would be lower owing partially to anatomic variations of the venous system in whom this approach might not be feasible. Furthermore, cubital venous access is limited by the smaller size (radius) of the catheters used to deliver local thrombolysis. Likewise, current ESC guidelines for PE support CDT-based treatment with only class IIa indication in high-risk PE while the same level is endorsed for the intermediate-to-low risk PE but only in cases in which hemodynamic deterioration occurs despite therapeutic anticoagulation. Similarly, this study lacks a direct comparison of the femoral or jugular versus cubital vein approach with respect to treatment safety and efficacy outcomes in this patient population. Equally so, cubital access intervention could be easily tested against placebo/sham control in a randomized fashion and this work might lay a foundation for such randomized controlled trials in the future. However, this study was not designed for such comparisons but rather it was devised as a proof-of-concept study with the primary objective of demonstrating the feasibility and safety of the CDT through a superficial cubital vein by using readily available equipment in the cath lab for the purpose of treating patients with intermediate and high-risk acute PE.
Conclusions
Based on our consecutive pilot cohort it was demonstrated that CDT using the cubital venous approach is a technically simple, safe, and feasible treatment option for the intermediate-to-high risk PE. It might especially be suited for patients that are clinically deteriorating and/or those with a high risk of major systemic bleeding that requires immediate clot lysis. We believe that the “first-cubital approach”, in experienced centers, has the potential to become the first-line treatment if CDT is indicated for the acute PE. However, further research is mandatory to elucidate the potential benefits in comparison with the femoral and/or jugular approach or sham control to validate these initial findings.








