Fibrolamellar hepatocellular carcinoma (FL-HCC) was first described by Edmonson in 1956 as an adult type of liver tumour in a 14-year-old female with no underlying liver disease [1]. Despite FL-HCC being a variant of hepatocellular carcinoma (HCC), it differs from HCC in that FL-HCC most commonly affects younger patients (10–35 years) without underlying liver disease. FL-HCC patients are more likely to be female [2]. FL-HCC often presents with abdominal pain, nausea, abdominal fullness, malaise, and weight loss. Rarely, patients present with symptoms related to tumoural hormone production, such as gynaecomastia resulting from aromatization of androgens, or paraneoplastic production of thyroid hormones or β-human chorionic gonadotropin (β-hCG) [3, 4].
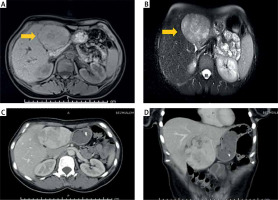
A 32-year-old female patient was admitted to the internal medicine service with complaints of recurrent deep vein thrombosis, left leg/left arm thrombosis, and pulmonary embolism with pancytopenia. Due to thromboembolism, the diameter of her left leg was larger, and anticoagulant treatment was started. Blood examination revealed elevated D-dimer (1612 ng/dl) and normal bilirubin, prothrombin time, thrombocyte, and α-fetoprotein. Collagen tissue diseases were suspected in this young female patient due to recurrent thromboembolism. Anti-nuclear antibodies (ANA), paroxysmal nocturnal haemoglobinuria (PNH) panel, the von Willebrand factor cleaving protease ADAMTS13, and thrombophilia panel were negative. Phospholipid antibodies were negative, antithrombin-3, protein C, and protein S were within normal range, but lupus anticoagulants (LA) were positive. LA were retested for the control, and this time they were negative. In addition, the level of vitamin B12 in the blood was 84 pg/ml, which is below the normal range (157–883 pg/ml). Because collagen tissue diseases and hereditary thrombophilia were ruled out, an underlying malignancy was suspected, due to the absence of collagen tissue diseases and hereditary thrombophilia. Abdominal CT (Figures 1 A, B) and MRI (Figures 1 C, D) were performed. A 100 × 82 × 54 mm lobule heterogeneous mass with nodular calcifications from segments 2 to 3 was detected in the left liver lobe. Fine needle aspiration biopsy was performed with EUS. The biopsy showed polygonal eosinophilic cells. Their cytoplasm was densely granular or foamy with the presence of granules and pale bodies. Tumour cells were positive for arginase and hepatocyte paraffin-1 (HepPar-1); both proteins are helpful biomarkers for HCC detection. These findings conducted us to FL-HCC. Left hepatectomy with hepatoduodenal lymph node dissection was carried out. The postoperative course was uneventful, and she was discharged on the 6th postoperative day. The pathology report confirmed a 10 × 7 × 5 cm mass with clear surgical margins, pT1N0 fibrolamellar FL-HCC (Figure 2). Immunohistochemistry staining was as follows: Heppar (+), pCEA (+), Cytokeratin 7(+), Arginaz (+), Glypican 3 and Glutamine synthetase (+), Cytokeratin 19 (–), and Cytokeratin 8-18 (–). She discontinued her anticoagulant treatment after discharge, and no thromboembolic attack occurred during the 30-month follow-up period.
In our case, the patient presented with recurrent eccentric thrombosis and pulmonary embolism due to paraneoplastic syndrome. To our knowledge there are only 2 cases of FL-HCC that presented with such symptoms. One of them is a 65-year-old female patient who presented with recurrent deep vein thrombosis [5], and the other case is a 16-year-old girl who presented with thrombophlebitis [6]. This unusual presentation may lead to misdiagnosis or late diagnosis; as a result, the patient may miss the opportunity of surgical treatment for a potentially resectable tumour that gradually progresses to an incurable stage.
Figure 1
A, B – CT image of FL-HCC: 100 × 82 × 54 mm lobule heterogeneous mass with nodular calcification from segments 2 to 3 in left liver. C, D – MRI image of FL-HCC: Hyperintense mass with hypointense fibrous central scar on T2-weighted images
MRI can be helpful in distinguishing FL-HCC from other liver lesions. FL-HCC tumours are usually hypointense on T1-weighted images and hyperintense T2-weighted images with a fibrous central scar that remains hypointense on both T1- and T2-weighted images. The hypointensity of the central scar can help differentiate FL-HCC from benign liver masses such as focal nodular hyperplasia, which typically has a hyperintense central scar [7]. FNA may not be required for diagnosis in a case of cirrhotic liver if the liver mass is compatible with HCC by contrast enhancement patterns. However, if the liver is not cirrhotic, FNA can help confirm the diagnosis and is recommended by the American College of Gastroenterologist Guidelines [8]. In this case fine needle biopsy was performed preoperatively, and the result was consistent with FL-HCC.
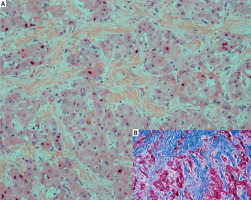
Up to 75% of FL-HCC cases have a central stellate scar and prominent fibrous tissue and are characterized by large polygonal or spindle-shaped tumour cells with deeply eosinophilic cytoplasm due to abundant mitochondria and prominent nuclei arranged in cords surrounded by lamellate collagen fibres (Figure 3). There is no cirrhosis in the surrounding liver parenchyma [9]. FL-HCC often stains strongly for cytokeratin 7 (CK7) and epithelial membrane antigen, which are characteristic of biliary differentiation as well as markers of hepatic differentiation like HepPar-1.
Figure 3
A – HE-stained section of FL-HCC. Large polygonal tumour cells with oncocytic appearance embedded in fibrolamellar stroma (HE, magnification: 200×). B – Masson-Trichrom stain also highlights the fibrolamellar fibrosis (inner right)
Aggressive surgical resection is the mainstay of treatment for potentially resectable FL-HCC. Patients undergoing resection may also benefit from concomitant regional lymphadenectomy [10]. While the 5-year survival rate of patients who underwent FL-HCC resection is 70%, this rate is 0% in patients who do not undergo surgical resection. Complete resection is not always possible, but it is important for survival. Long-term overall survival is associated with R0 resection. Several factors, such as younger age, earlier tumour stage at diagnosis, and the absence of large vessel invasion or thrombosis are associated with a better prognosis following surgery. Poor prognosis includes lymph node metastasis, multiple tumours, metastatic disease at presentation, and vascular invasion [11]. Fortunately, our patient was diagnosed at an operable stage despite approximately 1 year of research from the onset of thrombosis symptoms to diagnosis. R0 resection was successfully performed before lymph node metastasis or large vessel invasion occurred. After left hepatectomy the patient has had no new thromboembolism developing in the 2.5-year follow-up.
FL-HCC is a rare tumour that is important to differentiate from FNH, hepatic adenoma, and classic HCC. Complete surgical resection with lymphadenectomy represents the best chance for long-term survival. FL-HCC is often an asymptomatic disease, but it may present with unusual symptoms, as in our patient. Recurrent deep vein thrombosis, upper extremity thrombosis, and pulmonary embolism are rare presentations of FL-HCC, which must be kept in mind for patients having these symptoms but who are negative for genetic thrombophilia and collagen vascular disorders. FL-HCC’s propensity for thrombosis may be an unknown/little-known paraneoplastic syndrome that needs further investigation. Some hormones or enzymes like ‘arginase’ may be responsible for the deep venous thrombosis.











