Introduction
The development of laparoscopic surgery over the last 30 years has allowed for the widespread use of this technique in colorectal surgery [1, 2]. Laparoscopic surgery of colorectal cancers allows one to reduce the risk of complications in the postoperative period and reduce the length of stay of patients while maintaining comparable long-term results [3–5]. One of the most serious complications observed in the postoperative period is a leak in the intestinal anastomosis. Anastomotic leakage (AL) after laparoscopic procedures does not occur more often than after open surgery, but increases perioperative mortality, extends the time of hospitalization of patients, reduces the quality of life, and increases the costs of treatment [6–8]. Due to the lack of a uniform definition of leakage, the frequency of this complication in various studies ranges from 1 to 19% [9, 10]. The occurrence of AL depends on many factors such as age, gender, ASA score, malnutrition, obesity, smoking, stage of cancer, radio- and chemotherapy history, intraoperative blood loss and the duration of the procedure. The type and location of the anastomosis are important [11, 12]. The reported incidence of AL is 1–4% for ileocolic anastomoses, 2–3% for colocolic anastomoses, 3–7% for ileorectal and 5-19% for colorectal/coloanal anastomoses [13]. The main role in the formation of leakage from the anastomosis is attributed to disorders of blood supply and insufficient perfusion of the integrated intestinal sections. Intraoperative assessment of blood supply based on color control and bleeding from the resection margins is inaccurate and often underestimates the risk of leakage [14, 15].
Intraoperative fluorescent angiography (FA) using indocyanine green (ICG), thanks to the use of laparoscopic equipment equipped with special electronic filters, is a method that allows one to assess the blood flow through the composite sections of the intestine and thus prevent the development of postoperative anastomotic leakage [16, 17].
Indocyanine green is a non-toxic dye and has been used in medicine since the 1950s, initially in the evaluation of cardiac ejection and liver function, and then in the evaluation of retinal flow. It was authorized by the FDA for clinical use in 1956. After administration, fluorescence of indocyanine green can be observed by using near-infrared light with a wavelength between 750 and 800 nm with appropriate cameras. Indocyanine green is broken down in the liver and the half-life is 3–4 min. This allows for multiple administration of the dye in one operation [18].
Aim
The aim of the study is to present the preliminary results of the use of intraoperative fluorescence angiography using indocyanine green during rectal resection procedures due to cancer in the prevention of anastomotic leakage.
Material and methods
A prospective, randomized, controlled, single center clinical study was carried out with the consent of the local bioethics committee (1072.6120.126.2020). The study was registered at ClinicalTials.gov. (NCT05263336). All patients included in the study gave their informed consent to participate in it.
The number of patients was estimated based on the primary endpoint of the study, i.e., the rate of anastomotic leak. The number of patients was set so that the power of the test was at least 80% with a significance level of < 0.05. To obtain such parameters, 78 patients in each group should be included in the study. We are currently publishing data in the middle of our study.
Both sexes aged 20–80 years old were included in the study, with potentially resectable rectal cancer located on colonoscopy up to 12 cm from the rectal margin, operated on in a planned manner, in which laparoscopic rectal resection with the formation of intestinal anastomosis was planned. Qualification for preoperative radio- or chemoradiation was based on magnetic resonance imaging (MRI) assessment. Patients were operated on 5 to 7 days after short radiotherapy and 5 to 6 weeks after chemoradiation.
The exclusion criteria were the lack of consent to participate in the study, allergy to any component of the drug, the inability to perform laparoscopic surgery, and emergency surgery. Additionally, patients for whom an intraoperative decision was made not to perform anastomosis were excluded from the study.
Patients qualified for laparoscopic surgery were randomly assigned to one of two groups. Randomization of patients assigned to groups took place during surgery.
The study group consisted of patients who underwent intraoperative evaluation of intestinal perfusion at two moments of surgery – before the choice of the anastomosis site, and after the anastomosis. The evaluation was performed by a surgeon after intravenous administration of 1 ampoule (50 mg) of ICG diluted with 10 ml of water for injection and divided into two equal doses (Verdye 5 mg/ml, Diagnostic Green). The first dose of dye was administered intravenously after intestinal dissection and allowed for choosing the sites of intestinal intersection. The second dose of dye was administered intravenously after the anastomosis to assess the blood supply of the edges of the anastomosis (Photos 1–3).
Photo 1
Identification of the ischemic bowel during laparoscopic anterior resection – standard light (note no difference in the two segments)
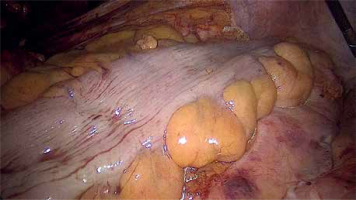
Photo 2
Identification of the ischemic bowel during laparoscopic anterior resection using near infra-red light after injection of ICG (difference between two segments)
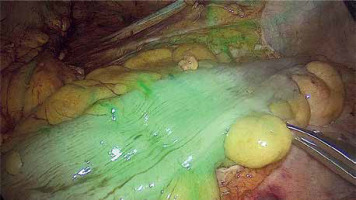
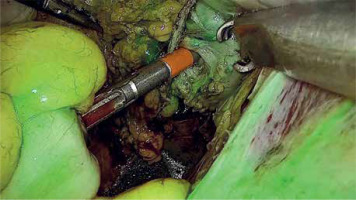
Photo 3
Assessment of bowel perfusion before anastomosis using near infra-red light after injection of ICG
The control group consisted of patients who were operated on using the same technique as the study group, but without the use of intraoperative fluorescent angiography, in which the evaluation of intestinal blood supply was carried out in visible light.
In both groups, the length of hospitalization, the frequency of complications, the severity of complications according to the Clavien-Dindo classification [19], the frequency of anastomotic leaks, and the need for reoperation were assessed.
The primary endpoint, and at the same time the measure of the effectiveness and impact of the use of indocyanine green in intestinal anastomosis, was the number of complications in the form of anastomotic separation.
The secondary endpoints were the length of hospitalization, the need for reoperation and the emergence of a stoma.
The operated patients were managed in accordance with the ERAS protocol. In all patients, mechanical preparation of the intestine for the procedure was applied. All operations were performed laparoscopically. All operations were performed using the 4K Karl Storz laparoscopic system. High inferior mesenteric artery ligation was used. Mobilization of the splenic flexure and descending colon and partial or total mesorectal excision depended on the location of the tumor and anatomical conditions. The exteriorization of the tumor was carried out by mini-laparotomy above the pubic symphysis from the Pfannenstiel cut. The evaluation of the ends of the intestine to be anastomosed was carried out in the study group using fluorescent angiography using ICG, and in the control group only based on macroscopic assessment by the operator and the assistant. If inadequate blood supply was found, ischemic sections of the intestine were resected proximally. The anastomosis was performed using the two-stapler technique, using Echelon Flex, Ethicon staplers and Medtronic 28–29 mm circular staplers.
Evaluation of postoperative patients for anastomotic leaks was performed up to 30 days postoperatively. An anastomotic leak was defined according to the International Study Group of Rectal Cancer as any anastomotic defect leading to communication between the intestinal lumen and the external space [20]. The occurrence of symptoms of peritonitis, the appearance of intestinal content in the drain or wound, the presence of rectovaginal fistula, and the result of rectal or endoscopic examination were considered.
Clinical data on the course of treatment have been obtained from the hospital information system. The obtained data were collected in an electronic database.
Statistical analysis
Statistical analysis was performed using the SPSS statistical package by IBM. Quantitative results are expressed in average with standard deviation. Student’s t-test and the non-parametric Mann-Whitney U test were used to analyze quantitative results. For the evaluation of the qualitative variables, the χ2 test was used. The Spearman correlation test between two variables was used as well. The level of statistical significance was p < 0.05.
Results
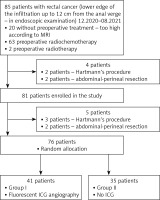
From December 2020 to August 2021, 85 patients with rectal cancer were initially included in the study. On endoscopic examination, the lower limit of the tumor was up to 12 cm from the anus verge. Based on the MRI study, 63 patients were referred for preoperative radiochemotherapy and 2 patients for radiotherapy. 20 patients due to the too high position of the tumor in the rectum did not receive neoadjuvant treatment. After further analysis of the results of the studies, another 4 patients, who were planned for surgery using the Hartmann procedure or abdominal-perineal amputation, were excluded from the study. Eighty-one patients were included in the study. Intraoperatively, due to the degree of tumor staging or anatomical conditions, it was decided not to perform anastomosis in 5 cases. Finally, 76 patients who underwent anastomosis were randomized intraoperatively. Forty-one patients (group I) were randomized to receive fluorescent angiography using ICG, and 35 patients (group II) were randomized to a group where, prior to the anastomosis, blood supply was not assessed using fluorescent angiography (Figure 1).
The two groups did not differ significantly in terms of age, gender, body mass index (BMI), ASA score, disease stage, tumor location or neoadjuvant treatment. A precise comparison of the groups in terms of these parameters is presented in Table I.
Table I
Demographic and clinical characteristics of patients
All patients underwent laparoscopic anterior rectal resection with partial or total mesorectal excision. In no case was a protective ileostomy performed. Groups I and II did not differ in the average distance of the anastomosis from the anal verge: 5.6 cm and 5.7 cm, respectively (p = 0.983). The duration of the operation was 132.4 min in group I and 128.6 min in group II. These differences were also not significant (p = 0.676). During surgery, the contrast time of the intestinal wall after administration of indocyanine green was 22 to 65 s, on average 42.2 s. The average time needed to perform fluorescent angiography was 4.3 min, which represented only 3.2% of the duration of the procedure. In 3 (7.3%) cases, based on the angiography, ischemia of the sections of the intestine prepared for anastomosis was found and it was necessary to broaden the scope of resection. In group II, based on the traditional assessment of blood supply, no patient’s anastomosis was changed.
Complications in the postoperative period occurred in 6 patients from group I (14.6%) and 7 from group II (20%). In both groups, Clavien-Dindo class I and II complications prevailed. There were no complications with the dye. The studied groups did not differ in terms of the occurrence of such complications as temporary gastrointestinal motility disorders, surgical site infections and dysuria.
In the study group, after angiography, 3 (7.3%) patients underwent widening of the extent of resection to achieve optimal blood flow in the anastomosed bowel stumps. No anastomotic leakage was observed among these patients. In the group without the use of ICG, due to the absence of observed signs of poor blood supply to the anastomosed bowel stumps, the extent of resection was not widened in any case. However, the difference between the two groups in modification of the anastomotic site was not statistically significant.
In group I, there was no case of anastomotic leakage, in contrast to group II, where there were 3 (8.6%) cases of anastomotic leakage. However, this difference did not reach statistical significance (p = 0.093). 2 patients, due to developing peritonitis, required emergency surgery with colostomy. In 1 case, the AL was endoscopically treated. No deaths due to leakage have been observed.
The studied groups did not differ significantly in terms of neo-adjuvant treatment. There was also no effect of preoperative radio-chemotherapy on the risk of anastomotic leakage.
A detailed comparison of the groups in terms of these parameters is presented in Table II.
Table II
Results of surgery in the two groups
A significant factor affecting the occurrence of anastomotic leakage was the duration of surgery: the longer the operation, the higher the risk of anastomotic leakage (p = 0.029) and prolonged hospitalization (p = 0.026).
Patients’ hospitalization time was longer in group II (mean: 4.9 days) than in group I (mean: 4.4 days), but the difference was not statistically significant.
Discussion
Laparoscopic colorectal surgery is now the standard treatment for colorectal cancer. With comparable long-term outcomes to open surgery, it allows for fewer complications and shorter hospitalization of the patient. However, laparoscopic surgery does not significantly change the risk of anastomotic leakage. The occurrence of this complication often requires reoperation, increases perioperative mortality, prolongs hospitalization, and increases treatment costs. It also affects the long-term results of treatment. Traditional methods of assessing anastomotic blood supply are subjective and cannot always be used in laparoscopic surgery.
A fairly common way to reduce the risk of anastomotic leakage is to create a diverting ileostomy. Studies published by Ihnát et al. and Niu et al. as well as a meta-analysis by Ahmad et al. show that creating a protective ileostomy is not an optimal solution either. It does not eliminate the possibility of anastomotic leakage, but only reduces its symptoms while increasing the risk of other postoperative complications, especially those dependent on the stoma. Additionally, temporary ileostomy decreases the quality of life of patients and requires reoperation to restore the continuity of the gastrointestinal tract, which may cause further complications [21–23]. For these reasons, we do not perform diverting ileostomies as standard at our institution, and no patient included in the study had one created.
The desire to reduce anastomotic leakage after colorectal surgery has led to the introduction of fluorescent angiography using indocyanine green. Many publications indicate the positive effect of this technique on reducing the risk of leakage, but there are relatively few randomized, prospective studies or meta-analyses. This limits the ability to unequivocally recommend this method to prevent anastomotic leakage. Most studies show that indocyanine green fluorescence angiography improves the ability to correctly assess the blood supply to the anastomosed bowel segments. ICG administration is a safe procedure with virtually no side effects. It can be used in almost any patient, except in cases of allergy to iodine compounds, hyperthyroidism or extreme renal failure [24].
The use of ICG angiography in our study did not significantly increase the duration of surgery (132.4 min – ICG group vs. 128.6 min – no ICG group). Boni et al. [25] and Mizrahi et al. [26] also found no significant difference in the duration of surgery. In contrast to these results, Skrovina et al. found a significantly longer operation time with FA (242 vs. 219 min) [27]. The duration of surgery matters. In our study, we found that prolonging the duration of surgery significantly increased the risk of anastomotic leakage. This is also in agreement with the meta-analysis data of Qu et al., where prolonging the duration of surgery increased the risk of AL [9].
Correct assessment of blood supply allows the intraoperative decision to resect the ischemic part of the intestine and perform anastomosis in a site with normal perfusion. Jafari et al. in the PILLAR II study noted a change in management strategy in 7.9% of cases. AL was not found in any of these patients [28]. The need to reposition the anastomotic line occurred in the study by Wada et al. in 16.1% of cases. However, this did not prevent leakage in 22.2% of these patients [29]. In this respect, our study is closer to the PILLAR II study. In the study group, after angiography, 3 (7.3%) patients underwent a widening of the extent of resection to achieve optimal blood flow in the anastomosed bowel segments. No anastomotic leakage was observed among these patients.
The main goal of introducing LA with ICG in colorectal surgery was to reduce the number of intestinal anastomotic leaks. The results of the meta-analysis by Li et al. [30] and Blanco-Colino et al. [31] show that the number of anastomotic leaks is significantly lower in the group of patients operated on with ICG. In our study the preliminary results suggested the possibility of such a relationship, but it did not reach statistical significance (p = 0.093). However, no patient who underwent intraoperative LA had anastomotic failure. Alekseev et al. in a randomized study found a significant reduction in the number of leaks for low colorectal anastomoses (4–8 cm from the crestal line), but no such relationship for anastomoses at a depth of 9–15 cm [32].
The hospitalization time of patients after laparoscopic rectal resections using fluorescence angiography was not significantly shorter compared to patients who underwent anastomosis without this technique. This is consistent with the results obtained by De Nardi et al. [33] and Alekseev et al. [32], who also found no such relationship. In contrast to these results Impellizzeri et al. and Otero-Piñeiro et al. observed a reduction in hospitalization time for patients operated on with FA [34, 35].
The design of the study, which was prospective and randomized, limited the number of patients included in the study. Despite the random allocation of patients to the study and control group, their relatively small number does not allow the elimination of bias. Another limitation may be subjective assessment of ICG without perfusion quantification and the need of use of laparoscopes with the highest, 4K, resolution available today, with electronic overlay of the fluorescence angiography image over the white light image, not available in most operating rooms.
Conclusions
Based on the results of our study, we can conclude that intraoperative fluorescent angiography using indocyanine green is a safe and effective method of assessing intestinal blood supply. Administration of indocyanine green does not increase the number of complications and does not significantly prolong the time of surgery. Our study suggests that the use of fluorescent angiography during laparoscopic rectal resections, by modifying the treatment plan, reduces the number of anastomotic leaks and reduces the length of the patient’s stay in the hospital. In our opinion, the introduction of fluorescence angiography using ICG as a standard method of assessing anastomosis in colorectal surgery is justified.
It is necessary to continue the study until the planned number of patients is reached. This may allow the statistical significance of the results to be reached and final conclusions to be drawn.