Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent cancers worldwide. It carries a poor cancer-specific survival despite the recent advances in its diagnosis and treatment [1]. HCC development is usually associated with inflammation of the liver parenchyma that may be caused by several factors including viral hepatitis, nonalcoholic steatohepatitis (NASH) and alcohol steatohepatitis (ASH) [2]. Exposure of liver cells to repetitive injuries initiates a process of tissue and cell repair in order to restore the normal liver anatomy and function. However, continuous exposure to injurious factors leads to consecutive cycles of hepatocyte destruction-regeneration which promotes HCC tumorigenesis [3]. The HCC microenvironment comprises several cellular and non-cellular components including stromal cells, endothelial cells, immune cells, inflammatory cells, cytokines and extracellular matrix, and this microenvironment has a crucial role in HCC initiation and progression [4].
A proinflammatory cytokine called macrophage migration inhibitory factor (MIF), which is mostly generated by Th2 cells and macrophages, can mediate the response to stress and infection by activating innate and adaptive immunological pathways [5]. MIF, a 12 kDa peptide with 114 amino acids, is encoded by a single gene on human chromosome 22q11.2m [6]. It primarily interacts with its receptor CD74 to join forces with CD44 to create a complex, which may last long enough to activate the ERK-MAPK pathway through the signal transduction of Src tyrosine kinase. The translocation of NFκB to the nucleus, upregulation of PLA2 and prostaglandin, and arachidonic acid pathway stimulation are some of the pathway’s downstream effects [7]. MIF-173G/C, a G to C transversion inside the MIF promoter region at position -173 which provides an AP4 transcription factor binding site, is the only single-nucleotide polymorphism discovered thus far in the 50 regions of the MIF gene [8]. MIF-173G/C polymorphism was linked to cancer susceptibility, according to previous research [9]. MIF has the potential to promote tumor growth and tumor-associated angiogenesis in mice [10]. So, its role in HCC is not clearly explored. Therefore, the current research aimed to test whether MIF gene polymorphism could be considered as an additional risk factor for development of HCC among a cohort of Egyptian patients with chronic viral hepatitis (hepatitis B virus [HBV] or hepatitis C virus [HCV]).
Material and methods
Study design, participants and data collection
The study protocol was prepared in accordance with the Declaration of Helsinki and has been approved by the Ethics Committee of the Faculty of Medicine, South Valley University, Qena, Egypt (code number. SVU-MED-MBC004-4-22-2-335). Written informed consent was obtained from each subject.
This study was a prospective randomized case-control study, carried out on 100 individuals recruited from the Departments of Internal Medicine, and Tropical Medicine and Gastroenterology, South Valley University Hospitals, Qena, Egypt between July 2021 and January 2022. The participants were categorized into two groups: group A included 50 patients with HCC on top of post-chronic hepatitis C (CHC) or post-chronic hepatitis B (CHB) liver cirrhosis; and 50 unrelated, age- and sex-matched healthy volunteers selected as controls formed group B. The study protocol was prepared in accordance with the Declaration of Helsinki and has been approved by the Ethics Committee of the Faculty of Medicine, South Valley University, Qena, Egypt. Each participant provided their written, informed consent. Sample size was adjusted to achieve 80% power and 5% confidence of significance (type I error).
Patients who were under the age of 18 years, those who had chronic liver disease other than CHC and CHB, and patients with liver metastasis or extrahepatic primary malignancies were excluded from the study.
Thorough medical history taking and full clinical examination were done. The diagnosis of HCC was based on the American Association for the Study of Liver Diseases (AASLD) guidelines [11]. All patients with HCC were evaluated for severity of liver disease using a Child-Pugh score [12], and tumor staging was assessed using tumor (T), nodes (N), and metastases (M) (TNM), Barcelona Clinic Liver Cancer (BCLC) scoring systems [13].
Investigatory workup
Biochemical and genetic analyses
1. Blood samples and biochemical assays
A 5 ml venous blood sample was obtained from each participant under sterile conditions; 2 ml were placed into EDTA containing tubes for complete blood counts using Sysmex, and then stored at –80°C for later genetic analysis. The remaining 3 ml of each sample were divided into two parts; the first part was placed into citrated tubes and was centrifuged to obtain citrated plasma, while the second part was evacuated into serum gel separator tubes and was allowed to be clotted at 37°C for 30 min then was centrifuged at 3500 rpm for 10 min to obtain serum. Using an autoanalyzer (Dialab 450 system), the separated sera were used for assays of serum creatinine, liver function [albumin, total and direct bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP)]. Citrated plasma samples were used for assay of prothrombin time (PT), prothrombin concentration (PC), and international normalized ratio (INR). Serum α-fetoprotein (AFP) measurement was performed by microplate ELISA reader (EMR-500, Labomed Inc., Los Angeles, CA 90034 US) using a commercially available ELISA assay kit. Hepatitis B surface antigen (HBsAg) and HCV antibody detection were performed using the automated MiniVidas immune-assay system (BioMerieux, Marcy l’Etoile, France).
2. Single nucleotide polymorphism detection technique for MIF 173 G>C
DNA extraction: Performed using the G-spin total DNA extraction kit methodology (iNtRON Biotechnology, Inc., Korea). The extracted DNA was kept at –80°C for later genetic study;
MIF genotyping.
Beijing SBS Genetech, China provided the two particular oligonucleotide primers for MIF 173 G>C (rs755622): the forward primer 5’-ACTAAGAAAGACCCGAGGC-3’ and the reverse primer 5’-GGGGCACGTTGGTGTTTAC-3’, in keeping with earlier protocols [14, 15], using polymerase chain reaction-restriction fragment length polymorphism (RFLP-PCR).
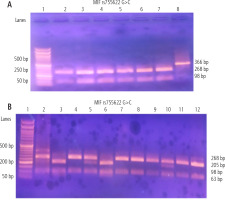
The following PCR settings were followed after combining 12.5 µl of the PCR master mix solution (Catalog no. 25028, iNtRON Biotechnology, Korea) with 1 µl of the forward primer, 1 µl of the reverse primer, 8.5 µl of nuclease-free water, and 2 µl of extracted DNA for a total volume of 25 µl: 35 cycles were performed using the Biometra thermal cycler (Serial no. 2603204, Biometra, Germany) at the following temperatures: 94°C for 30 seconds, 60°C for 30 seconds, 72°C for 60 seconds, and a final extension of 10 minutes. The restriction enzyme FastDigest Alu1 (FD0014, Lot: 00147479, Thermo Fisher Scientific) was used to digest the PCR products (Fig. 1A), which were 366 bp in size. A 50 bp DNA ladder (Catalog No. 24072, iNtRON Biotechnology, Korea) was applied. Ten microliters of the PCR reaction mixture were added to 2 µl of 10X buffer, two microliters of the restriction enzyme, and 18 µl of nuclease-free water. 2% agarose gel stained with 5 µl of ethidium bromide and a UV transilluminator were used. The wild genotype (GG) variant had two MIF restriction fragments of 98 and 268 bp, the homozygous (CC) mutant genotype had three fragments of 205, 98, and 63 bp, and the heterozygous (GC) mutant genotype had four fragments of 268, 205, 98, and 63 bp (Fig. 1B).
Fig. 1
Gel electrophoresis of the PCR products and MIF rs755622 G>C SNP using PCR-RFLP method; numbers refer to lanes. A) Lane 1 shows 50 bp DNA ladder; lanes 2-7 represent wild genotypes (GG) with 268, 98 bp bands; lane 8 shows amplified DNA segments (undigested PCR product) of length 366 bp. B) Lane 1 shows 50 bp DNA ladder; lanes 2, 5, 10 are heterozygous mutant (GC) genotypes with 268, 205, 98, 63 bp bands; lanes 3 and 6 are homozygous mutant (CC) genotype with 205, 98, 63 bp bands; lanes 4, 7, 8, 9, 11 and 12 are wild genotypes (GG) with 268, 98 bp bands
HCC diagnosis
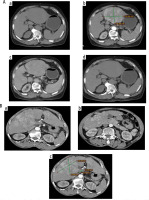
Multiphasic CT was conducted with a 64-MDCT scanner (Aquilion 64, Toshiba Medical, Ottawara, Japan). Images were obtained in the craniocaudal direction. Hepatic arterial phase scanning began 30-40 s after the injection of 120 ml of a non-ionic iodinated contrast agent (iopamidol; Iopamiro 300, Bracco) at a rate of 3-4 ml via a bolus-triggered technique. The contrast agent was administered through the antecubital vein with a power injector. The portal phase scanning began 70 seconds and equilibrium phases of scanning began 180 seconds after the injection of the contrast agent (Fig. 2A, B).
Fig. 2
A) 73-year-old male patient with cirrhotic changes in terms of irregular surface and deepened fissures with large left lobe hepatocellular carcinoma about 10 × 10.5 cm appearing hypodense in (a) non-contrast image and showing enhancement at the arterial phase in (b) and washing out at the portal phase and appearing completely hypodense in delayed CT image in (c, d). B) 63-year-old female patient with cirrhotic liver disease with multicentric hepatoma seen at both lobes; the largest is seen at the eighth lobe at segment VIII, about 9 × 10 cm, all showing enhancement at the arterial phase and porta hepatis lymph node about 1 × 2 cm
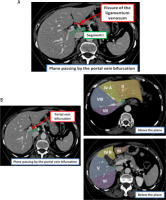
Localization of the liver HCC was based on the identification of three hepatic veins and the plane passing by the portal vein bifurcation. Segment I is defined by the portal bifurcation and the fissure of the ligamentum venosum (Fig. 3A). The left hepatic lobe is divided into segments II and III. The left lobe is divided into segment II above the plane passing by the portal vein bifurcation, and into segment III below (Fig. 3B). Segment IV is placed between the axis of the umbilical scissura on the left and the plane crossing via the middle hepatic vein on the right. This segment can be split into two smaller segments, upper IV A and lower IV B, by a line that runs through the left portal vein’s umbilical region (Fig. 3B). Segment VIII is the upper portion and segment V is the lower portion of the right anterior sector with respect to the plane passing via the portal vein bifurcation. The right posterior sector’s upper portion is segment VII, and its lower portion is segment VI (Fig. 3B).
Statistical analysis
Data entry and data analysis were done using SPSS Statistics version 19 (IBM). Data were presented as number, percentage, mean and standard deviation for parametric data. The χ2 test and Fisher’s exact test were used to compare qualitative variables. The independent t test was used to compare between two quantitative variables for parametric data. The odds ratio (OR) with 95% confidence interval (CI) was calculated. The studied SNPs followed the Hardy-Weinberg (HW) equation [16, 17]. The p-value < 0.05 was considered statistically significant.
Results
Subject characteristics
A total of 100 participants were included in this study, who were then assigned to two groups: group A (50 HCC patients) and group B (50 non-HCC participants). The mean age of patients was insignificantly higher in group A: 60.26 ±7.62 years vs. 57.94 ±9.76 in group B (p = 0.205), while the number of females was equal (12 patients) in both groups. Data regarding comorbid conditions, CHC and CHB status, imaging characteristic and stages of HCC patients are presented in Table 1.
Table 1
Clinical and imaging characteristics of the included HCC patients (n = 50)
Regarding the biochemical profile in both study groups, group A showed significantly higher ALT, AST, ALP, serum bilirubin, INR, AFP, creatinine, leucocytic, neutrophilic and monocytic counts, and lower serum albumin, hemoglobin, platelets and lymphocytic counts when compared to group B (Table 2).
Table 2
Routine laboratory data of the study groups
Frequency distribution of genes and alleles of MIF (rs755622 G>C) single nucleotide polymorphism among the study groups
As shown in Table 3 and Fig. 4, the comparison between the two study groups regarding MIF genotypes and allele frequencies showed significantly higher frequencies of wild genotype GG and heterozygous mutant genotype GC and lower frequency of homozygous mutant genotype CC in HCC patients versus controls (66%, 24% and 10% vs. 32%, 8% and 60% respectively, p < 0.001). Using the dominant model, genotypes GG + GC were more prevalent among the cases vs. controls (90% vs. 40%, p < 0.001, OR = 13.5, 95% CI: 4.569-39.889). On the other hand, using the recessive model, genotypes GC + CC were more prevalent among controls vs. cases (68% vs. 34%, p < 0.001, OR = 4.125, 95% CI: 1.792-9.497).
Table 3
Genotype and allele frequencies of MIF rs755622 G>C single nucleotide polymorphism among study groups
Fig. 4
Genotype (A) and allele frequencies (B) of MIF rs755622 G>C single nucleotide polymorphism among study groups
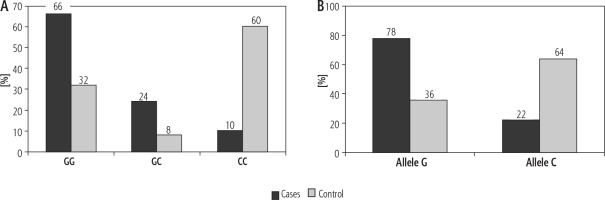
In addition, there was a significantly higher allele G frequency and lower allele C frequency among the patients with HCC compared to controls (78% and 36% vs. 22% and 64%, respectively; p < 0.001), indicating that G allele carries a risk of HCC development with OR = 6.303, CI: 3.374-11.775.
Genetic analysis of MIF (rs755622 G>C) in terms of various disease characteristics of hepatocellular carcinoma
Comparison between HCC patients regarding their stages of liver disease showed higher frequency of codominant genotype GG in patients with advanced Child scores B and C versus Child A, and TNM stages III and IV vs. stages I and II (p < 0.05 for both).
Although there was higher frequency of genotype GG in patients with BCLC stages C and D vs. stages A and B it did not reach a significant value (p > 0.05).
In addition, there was a significantly higher G allele frequency and lower C allele frequency among patients with advanced stages in comparison to early stages; Child B and C vs Child A, and TNM stages III and IV vs. I and II (p < 0.05 for both).
Although there was higher frequency of the G allele in patients with BCLC stages C and D versus stages A and B it did not reach a significant value (p < 0.05) (Table 4).
Table 4
MIF rs755622 G>C genotype and allele frequencies in terms of the different scoring systems of HCC
In patients with different HCC characteristics and stages, MIF genotypes and allele frequencies showed more prevalent codominant wild genotype (GG) among patients with multiple hepatic focal lesions (81.0%) compared to patients with a single hepatic focal lesion (81% vs. 55.2%, p = 0.074), while the homozygous mutant genotype CC was detected only in patients with a single hepatic focal lesion. In addition, there was a significantly higher G allele frequency and lower C allele frequency among the patients with multiple hepatic focal lesions compared to those with a single hepatic focal lesion (90.48% and 9.52% vs. 68.97% and 31.03%, respectively; p < 0.01).
HCC patients with nodal spread showed higher frequency of the wild genotype GG compared to those without nodal spread of their HCC (71.4% vs. 65.1%, p = 0.631), while the homozygous mutant genotype CC was only detected in patients with absence of intra-abdominal lymph node metastasis. In addition, there was higher allele G frequency and lower allele C frequency among the patients with intra-abdominal lymph node metastasis compared with the patients with absence of intra-abdominal lymph node metastasis (85.7% and 14.2% vs. 73.68% and 26.32%, respectively), but not reaching a significant level (p > 0.05) (Table 5).
Table 5
MIF rs755622 G>C genotype and allele frequencies in terms of the clinical characteristics of HCC
Additionally, there were no significant difference regarding MIF genotype and allele frequencies in patient with HCC after chronic HBV infection compared to those with chronic HCV infection (p > 0.05) (Table 6).
Table 6
MIF rs755622 G>C genotypes and allele frequencies in terms of viral hepatitis B and C status
Discussion
Hepatocellular carcinoma is the most common primary liver cancer and its increasing incidence and mortality make it a heavy global public health burden [18]. HCC development is a multistep heterogeneous process characterized by rapid progression and poor prognosis [19]. Despite the availability of many therapeutic options for HCC such as radical surgical resection, liver transplantation, local ablative therapies and trans-arterial chemoembolization (TACE), it has a high resistance to treatment and a poor overall outcome that could be attributed to the accumulation of several molecular and cellular alterations [20].
MIF is a well-known cytokine that is involved in many biological processes and plays a crucial role in the body’s endocrine immune system. Through inhibiting macrophage migration and promoting its settlement, proliferation, activation, and secretion at the site of inflammation, it plays a crucial role in the pathogenesis of various inflammatory, autoimmune, and even malignant diseases [21]. The gene encoding MIF in humans is located on the long arm of chromosome 22 (22q11.2) and has several polymorphic loci [22]. Previous studies demonstrated that MIF gene polymorphisms and MIF gene expression levels have a close association with the susceptibility, progression, response to therapy and outcome of many diseases such as sepsis, autoimmune diseases, cancer, obesity and diabetes [23, 24]. However, its particular relation with HCC development and progression has been an understudied issue until the time of this study, which aimed to highlight it in a cohort of Egyptian patients with HCC.
Multiple mechanisms could explain the role of MIF in HCC carcinogenesis, such as inducing tumor angiogenesis [25], enhancing proliferation and differentiation of tumor cells [26], promoting invasion and migration of HCC cells [27], influencing P53’s function or interacting with mutant P53, which determines the development of numerous malignancies in the human body [28].
Our results showed significantly higher distribution of genotypes GG and GC, and lower distribution of CC genotype in HCC patients compared to controls. Also, higher frequency of the G allele and lower frequency of the C allele were noted in HCC cases. Both findings suggest a significant association between genotypes GG + GC and the G allele with the possibility of HCC development.
A potential impact of MIF gene polymorphism on HCC progression and behavior was noted in this study, which showed higher prevalent of GG genotype and the G allele in patients with multi-focal HCC in comparison to those with a single lesion. Also, different distributions of MIF were detected in different stages of HCC; higher GG genotype and G allele frequencies were detected in patients with advanced HCC (BCLC stages C and D and TNM stages III and IV) compared to earlier HCC stages (BCLC stages A and B and TNM stages I and II, respectively). Interestingly, MIF polymorphism may play a significant role in HCC nodal spread as genotype GG and the G allele showed higher frequencies in HCC patients with lymph node metastasis compared to genotype CC and the C allele respectively.
MIF gene polymorphism rs755622 was significantly associated with an increased susceptibility to HCC in a study that included 202 patients with HCC (HCC group), 242 patients with chronic hepatitis B (CHB group), 215 patients with liver cirrhosis (LC group), and 227 healthy volunteers (control group). The G allele may be a significant risk factor for HCC. The MIF rs1007888 and rs2096525 polymorphisms, however, were not linked to HCC susceptibility (p > 0.05). Additionally, there was no correlation between MIF rs755622, rs1007888, or rs2096525 polymorphisms and susceptibility to CHB or LC [29]. A different earlier study [30] that only included CHC patients revealed a greater frequency of HCC with the C/C genetic polymorphism.
Conclusions
In the light of our results we concluded that there was a significant impact of MIF G/C gene polymorphism on the susceptibility and progression of hepatocellular carcinoma among the study population.
Study limitations
The main limitation was the relatively small sample size, so the findings of the current study have to be confirmed using larger scale studies. Lack of serum assays of MIF levels and the possible role of MIF gene polymorphism in response to therapy and recurrence among patients with HCC were additional limitations that could be taken into account in future studies.