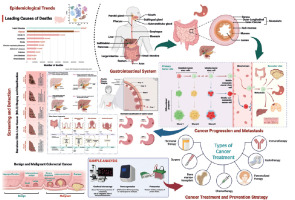
Introduction
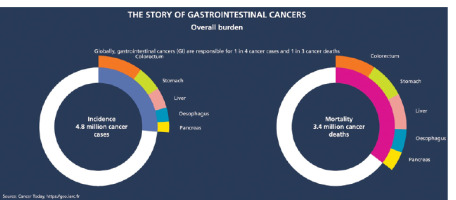
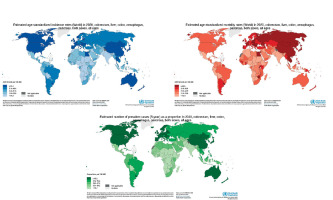
Gastrointestinal (GI) cancers are a major cause of morbidity and mortality worldwide, resulting in over 4.8 million new cases and 3.4 million deaths in 2020 alone [1, 2] (Figure 1). The most common GI cancers are colorectal, stomach, liver, oesophageal, and pancreatic cancer. According to the World Health Organisation, colorectal cancer (CRC) is the third most diagnosed cancer while stomach, liver, and oesophageal cancer are the 5th, 6th, and 7th most common cancers worldwide, respectively [3–5]. Pancreatic cancer also contributes significantly to cancer mortality, ranking 12th in global incidence but 7th in cancer mortality [2–7].
The distribution and trajectory of GI cancers vary significantly by geographic region, with less developed regions typically facing a higher burden compared to more developed countries [8, 9]. There are also differences in incidence, mortality, and temporal trends based on specific cancer sites within the GI tract (Figure 2). This review will provide a comprehensive examination of the global epidemiology and burden of major GI malignancies, including geographic, gender, and temporal differences. Prevention and early detection strategies to reduce the future burden of GI cancers will also be discussed.
Epidemiology of common gastrointestinal cancers
Colorectal cancer
Colorectal cancer is a significant contributor to cancer incidence and mortality worldwide. CRC accounts for approximately 10% of all cancer cases and deaths globally [1–3]. In 2020, there were an estimated 1.9 million new cases and 935,000 deaths from CRC worldwide (Table I). CRC is the 3rd most common cancer in men and 2nd most common in women. Incidence rates are highest in Australia/New Zealand, Europe, and North America, with rates generally higher in men than women in most regions. Mortality rates for CRC parallel incidence rates geographically (Table II) [3–5].
Table I
Covering key details on different gastrointestinal cancer types: mechanisms and risk factors, screening, treatment, and prognosis by stages
| Specific GI cancer | Mechanisms and risk factors | Screening | Treatment | Prognosis by stage |
|---|---|---|---|---|
| Liver cancer | ||||
| Stomach cancer | ||||
| Colon cancer | ||||
| Pancreatic cancer | ||||
| Oesophageal cancer | ||||
| Gastric cancer | ||||
| Colorectal cancer |
Table II
Global incidence and mortality for major gastrointestinal cancers. Data source: Global Cancer Observatory, International Agency for Research on Cancer 2020
From a temporal perspective, incidence and mortality rates rose for much of the 20th century in economically transitioning countries as diets became more “Westernised” and obesity rates increased [7]. However, rates have been declining in the United States, Australia, and Western Europe over the past few decades, predominantly due to increased screening and removal of precancerous polyps (Figure 3) [8]. In contrast, CRC incidence and mortality have been rising in countries that have experienced recent economic development and adoption of cancer-associated lifestyle factors like smoking, physical inactivity, and dietary changes (Table III).
Figure 3
Estimated numbers of new cases, incidence (A, B), prevalence (C), and mortality (D) of major gastrointestinal cancers to global cancer cases in 2020. Source: GLOBOCAN 2020
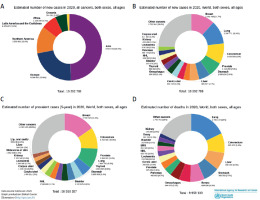
Table III
Risk factors for common gastrointestinal cancers. Data sources: Coghill AE et al. Nat Rev Gastroenterol Hepatol 2021; WCRF/AICR Diet, Nutrition, Physical Activity and Cancer: a Global Perspective. Continuous Update Project Expert Report 2018
There are also differences in CRC incidence and mortality based on anatomical location within the colorectum. Proximal colon cancer tends to be more common in women, while distal colon and rectal cancer is more frequent in men [4]. Rectal cancer incidence has been decreasing more rapidly than colon cancer incidence in the United States and other high-income countries [8, 9]. However, colon cancer mortality is declining faster than rectal cancer mortality.
Stomach cancer
Stomach cancer, also known as gastric cancer, is the 5th most diagnosed cancer and 3rd leading cause of cancer deaths worldwide [1–3]. In 2020, there were over 1.09 million new stomach cancer cases and 768,000 deaths globally (Figure 4, Table II). The highest incidence rates are observed in Eastern Asia, Central and Eastern Europe, and South America. Stomach cancer is now relatively uncommon in the United States and Western Europe. Historically, it was once one of the most common malignancies in the US, but rates have declined steadily over the past century. The dramatic geographic variations are believed to reflect differences in prevalence of Helicobacter pylori infection as well as dietary factors like high salt and nitrate intake.
Figure 4
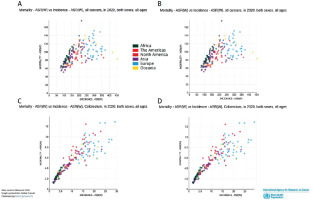
Contribution summary of Mortality ASR(W) vs incidence ASR(W) of all cancer’s vs GI cancers in 2020. Source: GLOBOCAN 2020
There is a marked male predominance, with incidence rates about twice as high in men than women in most populations. The male-to-female mortality ratio approaches 1.5 : 1. Temporal trends show substantial declines in stomach cancer incidence and mortality over the past 50 years in most world regions [7–9]. The exceptions are Southern and Eastern Africa, where rates have remained stable or increased slightly. The decreasing global rates are largely attributed to declining H. pylori infection, reduced consumption of preserved/salted foods, and improved nutrition and sanitation (Tables III, IV). However, obesity and tobacco use may attenuate the decline in some populations.
Table IV
Diagnosis and treatment of gastrointestinal cancers by stage. Data sources: Cancer Treatment Centers of America, American Cancer Society, Cancer Research UK
Liver cancer
Liver cancer is a highly lethal malignancy, resulting in over 830,000 deaths worldwide in 2020. It is the 6th most diagnosed cancer but the 3rd leading cause of cancer mortality [1–9]. Hepatocellular carcinoma (HCC) accounts for approximately 80% of primary liver cancers in adults (Table II). The incidence is highest in Eastern and South-Eastern Asia as well as Northern and Western Africa. The prevalence of chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections is a major contributor to geographic variations (Figures 5, 6). Most cases occur in less developed regions with high rates of HBV/HCV [1, 3, 9–11]. Other risk factors include aflatoxin exposure, heavy alcohol use, non-alcoholic fatty liver disease associated with obesity and diabetes, smoking, and hereditary haemochromatosis (Tables I–IV).
Figure 5
Age-standardised rate (world) per 100,000 with male/female and mortality with incidence combination including GI cancers with all cancers. Source: GLOBOCAN 2020
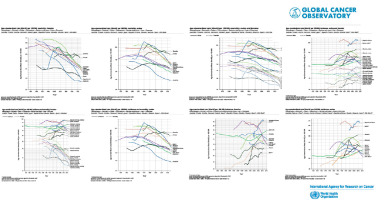
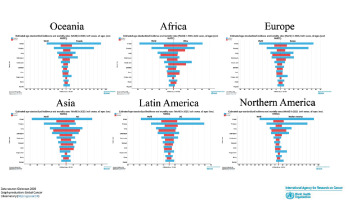
Figure 6
Estimated age-standardized incidence and mortality rates (world) vs. Oceania, Africa, Europe, Asia, Northern America and Latin America in 2020, both sexes, all ages. Source: GLOBOCAN 2020
Liver cancer disproportionately affects men, with incidence rates 2 to 4 times higher compared to women. However, the gender disparity is narrowing, particularly in low-risk areas like North America and Northern Europe where viral hepatitis is less endemic (Table III). On the temporal scale, the incidence is declining in some high-resource countries like Japan and Singapore that have implemented HBV vaccination programs [12]. Liver cancer mortality has also been decreasing in the United States, linked to better treatment of HCV. However, obesity-associated HCC is increasing and may slow the declines in liver cancer rates going forward.
Oesophageal cancer
Oesophageal cancer is the 7th most common cancer globally, with over 600,000 new cases estimated in 2020 [1–5]. The highest incidence rates are found in Eastern Asia and Eastern/Southern Africa (Table II). Oesophageal cancer exists in 2 main histologic forms: oesophageal squamous cell carcinoma (ESCC) and oesophageal adenocarcinoma (EAC). ESCC comprises 80% of cases globally and predominates in Asia and Africa, attributed largely to tobacco use and alcohol consumption (Tables III, IV) [12, 13]. In contrast, EAC predominates in North America and Europe, where its incidence has risen dramatically since the 1970s. This rapid rise is associated with increasing trends of obesity, gastroesophageal reflux disease (GERD), and Barrett’s oesophagus, a precancerous lesion. The male-to-female ratio approaches 3 : 1 for ESCC but is much lower for EAC (Table I).
On the mortality front, oesophageal cancer caused over 440,000 deaths in 2020, making it the 6th leading cause of cancer mortality. Mortality rates are highest in Asia and Africa [1–9]. Survival is poor, with 5-year relative survival under 20% in most world regions. However, survival is steadily improving in the United States and other high-income nations due to earlier diagnosis and improved treatments (Table IV). Reduced tobacco and alcohol use and prevention/treatment of obesity and GERD will be important in lowering the oesophageal cancer burden going forward.
Pancreatic cancer
Pancreatic cancer caused about 466,000 deaths globally in 2020, making it one of the most fatal cancers [1–5]. It is the 12th most commonly diagnosed cancer but the 7th leading cause of cancer death worldwide (Table II). The highest incidence rates are observed in North America and Europe, while mortality is equally high in both more and less developed regions (Tables II–IV). Known risk factors include tobacco smoking, obesity, diabetes mellitus, chronic pancreatitis, and hereditary cancer syndromes. The incidence is 50% higher in men than women in most populations [14].
On a temporal scale, pancreatic cancer incidence and mortality have been increasing in many world regions in parallel with rising rates of obesity and diabetes (Table III). The 5-year relative survival rate remains low, at just 11% in the United States and 7% in Europe (Figure 5). However, survival has been slowly improving, linked to both earlier diagnosis and improved therapies [15]. Preventing smoking and obesity, controlling diabetes, and new screening approaches for early detection will be important strategies for reducing pancreatic cancer burden in the future.
Regional variations
Beyond broad international patterns, there is heterogeneous distribution of GI cancer burden between and within world regions based on a variety of environmental, lifestyle, and genetic factors (Table III). Examining geographic variations can help identify populations most in need of preventive interventions and resource allocation.
Africa
The burden of GI cancers in Africa reflects both the distribution of risk factors like infectious agents and the availability of prevention and treatment resources. For instance, liver cancer incidence and mortality are highest in West Africa and other regions where hepatitis B virus infection is hyperendemic [1–7, 15]. Oesophageal squamous cell carcinoma is also common, linked to alcohol use and tobacco chewing in East Africa and Southern Africa. In contrast, CRC rates remain lowest in Africa, attributed to lower prevalence of obesity and Westernised diets. However, CRC incidence is rising in economically transitioning African countries like South Africa (Figures 7–9) [16, 17].
Figure 7
Estimated number of new cases, incidence, mortality, prevalence based on world population, both sexes, all ages in 2020. Source: GLOBOCAN 2020
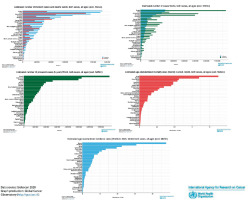
Figure 8
Estimated number of new real cases (million) from 2020 to 2040, both sexes, age (0–85+) with mortality (Africa + Latin America and Caribbean + Northern America + Europe + Oceania + Asia) based on different GI cancers. Source: Cancer Tomorrow 2023. Estimated number of new cases from 2020 to 2040, both sexes, age (0–85+) with mortality (very High HDI + High HDI + Medium HDI + Low HDI) based on different GI cancers. Source: Cancer Tomorrow 2023. Estimated number of new cases from 2020 to 2040, both sexes, age (0–85+) with mortality (Africa + Latin America and Caribbean + Northern America + Europe + Oceania + Asia) based on different GI cancers. Source: Cancer Tomorrow 2023

Figure 9
Estimated age-standardized incidence rates (World) in 2020 with different major GOI cancers, both sexes, all ages. Source: GLOBOCAN 2020
Stomach cancer has declined in much of Africa, though persistence of H. pylori infection and dietary nitrosamines maintain risk in certain populations. Resource limitations also contribute to poor GI cancer survival across much of Africa [18]. For example, 5-year CRC survival is less than 50% in Algeria versus over 65% in the United States. Expanded access to HBV vaccination, cancer screening, early detection, and treatment services are needed to reduce GI cancer burden in Africa [19].
Asia
Eastern Asia has among the highest incidence and mortality rates for stomach, liver, and oesophageal cancers globally [1–7]. This cancer pattern reflects the high prevalence of H. pylori and hepatitis viral infections [20]. For instance, China alone accounts for over 50% of liver cancer cases worldwide, linked to endemic HBV. While stomach cancer rates are declining in South Korea and other developed Asian nations, liver and oesophageal cancers remain common. Colorectal cancer is also rising rapidly in some Asian countries like Japan and Singapore that have adopted more Westernised lifestyles (Figures 7–9, Tables I–III) [21–23].
Central/South Asia have comparatively lower GI cancer rates, but the burden is increasing. For example, CRC rates are rising in India along with increasing life expectancy, obesity, and diabetes [24]. Prevention should focus on HBV vaccination, reducing tobacco and alcohol use, and curbing the Westernisation of diets. Early detection through endoscopy and stool testing are also being implemented in some Asian countries to improve GI cancer outcomes [25].
Europe
Colorectal cancer dominates in Europe, which has the highest CRC incidence globally along with Australia/New Zealand. Obesity, physical inactivity, smoking, alcohol, and Westernised diets underlie the high CRC burden [1–4, 26]. Stomach cancer remains common in Eastern and Central Europe, while liver cancer is rising due to hepatitis C infections and alcohol use. Oesophageal cancer is also prevalent in certain regions like Northern France, linked to alcohol consumption and possible nutritional deficiencies [27].
Implementation of CRC screening has helped lower incidence and mortality rates in much of Western Europe. Further reducing smoking and alcohol intake and increasing physical activity and nutrition are also important prevention targets. Refining organised screening and equalising access to optimal cancer care across Europe will further aid in alleviating GI cancer burden going forward [28].
North America
The United States has among the highest CRC incidence and mortality rates globally, reflective of widespread obesity, diabetes, and Westernised lifestyles. However, US CRC death rates have fallen by almost 35% from peak rates in the 1980s and 1990s due to increased screening [1–9]. Pancreatic cancer rates are also highest in North America, driven by rising obesity and diabetes. In contrast, stomach and liver cancer are less common, though liver cancer has risen with hepatitis C infections and obesity/diabetes. Oesophageal adenocarcinoma is also prevalent, associated with obesity and GERD (Tables I–III) [27–29].
There are disparities in GI cancer burden within North America related to race, ethnicity, and socioeconomic status. For instance, African Americans have the highest CRC mortality rates. Continuing to improve screening access and diet/lifestyle risk factor reduction are priorities to further lower GI cancer burden across all populations in North America.
South America
Stomach cancer remains highly prevalent in parts of South America like Brazil, Chile, and Colombia. H. pylori infection, tobacco use, and dietary factors (e.g. nitrates, salt) underlie the persistent burden [1–9]. Liver cancer is also common due to hepatitis virus spread and obesity. However, GI cancer rates are heterogenous across South America based on the distribution of risks [30]. For example, Argentina has comparatively high CRC rates linked to greater affluence and Westernised lifestyles. Improving access to H. pylori eradication, hepatitis prevention, and cancer screening/treatment across the socioeconomic spectrum can help alleviate GI cancer burden in South America [30, 31].
Australia/New Zealand
Australia and New Zealand have some of the highest CRC incidence and mortality rates worldwide, driven largely by high obesity prevalence and elements of Westernised lifestyle. More than 16,000 Australians are diagnosed with CRC each year [1, 9]. However, Australian CRC death rates have substantially declined since the 1980s through widespread screening implementation. Further improving healthy lifestyle behaviours and adherence to regular screening are priorities for driving down GI cancer burden in this region [14–16].
Prevention
Up to about 50% of GI cancer cases may be preventable through modification of environmental and lifestyle factors that increase cancer risk (Table III) [7–9]. Major primary prevention approaches include vaccination, chemoprevention drugs, behavioural changes like diet, treatment of pre-malignant conditions, and reducing population-level risk exposures (Table I).
Vaccination
Hepatitis B virus vaccination has emerged as a crucial cancer prevention tool, especially for reducing liver cancer incidence in highly endemic countries. The World Health Organisation (WHO) recommends that all infants receive the hepatitis B vaccine as soon as possible after birth, preferably within 24 h [1–4]. The vaccine provides over 90% protection from chronic HBV infection when given in infancy, which helps prevent development of cirrhosis and HCC. Universal HBV vaccination in Taiwan has reduced HCC incidence in children by nearly 90%. Widespread global implementation of HBV vaccination programs is a top priority for liver cancer prevention, especially in Asia and Africa where chronic HBV infection is highly prevalent [7–11].
Chemoprevention
Chemoprevention involves the use of natural or synthetic agents to inhibit, reverse, or retard development of cancer. Aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) have well-established CRC preventive efficacy (Tables I, IV) [1–3, 9]. Long-term daily aspirin reduces CRC incidence by 20–40% and mortality by about 35% in average-risk adults. The US Preventive Services Task Force recommends initiating low-dose aspirin as a chemopreventive for adults aged 50–69 years who have a 10% or greater 10-year cardiovascular disease risk and are not at increased risk of bleeding [12–15].
In people with Lynch syndrome or hereditary nonpolyposis colorectal cancer, aspirin reduces CRC risk by over 60%. The prostaglandin E2 inhibitor celecoxib also shows CRC preventive potential in high-risk groups when taken for at least 3 years [1–4]. However, adverse effects like GI bleeding and ulceration limit widespread aspirin and NSAID use for CRC prevention. Development of safer chemopreventive regimens remains an active research area.
Diet and lifestyle
Diet and nutrition play important roles in GI cancer development, with strong evidence linking excessive red and processed meat intake, high-fat diets, alcohol overuse, obesity, and salt-preserved foods to increased risk (Tables I–III) [7–13]. The World Cancer Research Fund and American Institute for Cancer Research recommend cancer prevention through consuming mostly plant-based diets, achieving/maintaining a healthy body weight, increasing physical activity, limiting red and processed meats, avoiding sugary drinks, limiting alcohol, and reducing salt and sodium intake [22].
Smoking is another major modifiable risk factor, responsible for a substantial proportion of stomach, pancreatic, and oesophageal cancers. Eliminating tobacco use would have a major preventive impact. Eliminating other carcinogen exposures like aflatoxins, asbestos, and radiation can help reduce GI cancer burden as well [18].
Treatment of pre-malignancy
Identifying and treating pre-malignant lesions or conditions offers opportunities to prevent cancers before they develop or intervene at early stages when they may be more successfully treated. For instance, early detection and removal of colorectal adenomas through endoscopic screening prevents progression to CRC [27]. Endoscopic surveillance and treatment of Barrett’s oesophagus similarly reduces risk of developing oesophageal adenocarcinoma.
H. pylori eradication may regress gastric atrophy and metaplasia, decreasing stomach cancer risk. Aspirin and NSAIDs also reduce incidence of oesophageal and gastric cancer in patients with Barrett’s oesophagus and gastritis, respectively (Table III) [11, 18]. Controlling obesity and GERD are other important strategies. Further research on optimal prevention and treatment interventions for pre-malignant conditions will support reducing GI cancer burden through early intervention.
Screening and early detection
Population-based screening allows for identification of GI cancers at earlier, more treatable stages and detection/removal of precancerous polyps or lesions. Most organisations recommend screening average-risk individuals for CRC beginning at age 45–50 years using stool-based tests, sigmoidoscopy, or colonoscopy (Table I) [1–6]. Colonoscopy is considered the gold standard, but resource limitations have led some national programs to use stool testing or flexible sigmoidoscopy. Modelling studies demonstrate that optimised CRC screening implementation could reduce incidence by 33–62% and mortality by 27–60%. However, effective program organisation and high population compliance are essential [2, 9].
The effectiveness of stomach cancer screening in high-risk areas like Japan and South Korea using barium swallow and endoscopy has also been well-established [1–6]. However, potential harms like false positives, cost-effectiveness, and optimal age ranges and intervals remain under study for potential organised stomach cancer screening programs. Limited evidence is available to support population-based oesophageal or liver cancer screening at present. However, endoscopic surveillance in higher risk groups may be warranted. Further research can help define optimal early detection approaches to reduce morbidity and mortality from GI cancers.
Conclusion on prevention
Primordial prevention through hepatitis B vaccination, reducing carcinogen exposures, and modifying lifestyle-related risks like smoking, poor diet, physical inactivity, obesity, and alcohol overuse can substantially lower GI cancer incidence. Secondary prevention through screening and early intervention for pre-malignant lesions and localised disease also offer opportunities to reduce GI cancer mortality in particular. A variety of prevention strategies will probably be needed, implemented in a tailored manner based on geographic variations in cancer sites, risk factor profiles, and resource availability. Continued research, policy changes, and health education will be crucial to enable broad roll-out of effective GI cancer prevention programs globally.
Treatment
Advances in cancer therapies have contributed to improved survival outcomes over recent decades for many GI malignancies. However, cure rates remain poor for most patients diagnosed with metastatic GI cancers. Earlier detection along with expanded access to optimal multimodality treatment is key to further improving GI cancer survival, especially in lower resource regions.
Colorectal cancer
Surgery is the cornerstone curative therapy for early-stage colon and rectal cancers. The standard of care also includes chemotherapy, either before (neoadjuvant) or after (adjuvant) surgery. Stage III colon cancer is treated with adjuvant multi-agent chemotherapy like FOLFOX (fluorouracil, leucovorin, and oxaliplatin) or CAPOX (capecitabine and oxaliplatin). Chemoradiation is typically recommended either pre- or post-operatively for stage II/III rectal cancers [1–4].
Targeted biologic agents like vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) inhibitors (e.g. bevacizumab, cetuximab) have also become part of the standard treatment protocols in stage IV metastatic colorectal cancer, improving response rates and survival when added to chemotherapy [6–9]. The integration of targeted therapy and immunotherapy into treatment paradigms will probably further advance the management of advanced CRC [11].
Despite advanced treatments, only 14% of metastatic CRC patients survive for 5 years, underscoring the importance of early detection. Adjuvant chemotherapy use varies globally based on resource availability [5–9]. One study found that just 30% of stage III CRC patients received adjuvant chemotherapy in some low-income countries versus over 80% in upper-middle- and high-income nations [9–11]. Improving access to surgical, chemotherapy, and radiation treatment capacity in limited-resource regions remains imperative to improving CRC outcomes worldwide.
Stomach cancer
Surgery is the only curative therapy for non-metastatic stomach cancer. Subtotal or total gastrectomy is performed based on cancer stage, often coupled with lymph node dissection. Chemotherapy or chemoradiation may be given before surgery to shrink the tumour or after surgery to eliminate microscopic disease. Targeted therapies like trastuzumab are added to traditional chemotherapy for HER2-positive metastatic disease [3–9].
Outcomes are generally poor, with 5-year relative survival under 30% globally. However, survival is over 60% when the cancer is diagnosed at an early localised stage, highlighting the value of early detection and treatment access. Post-operative chemoradiation utilisation and participation in clinical trials also positively impact survival but remain inconsistent across world regions [20–23]. Closing these treatment disparity gaps can aid in lowering global stomach cancer mortality.
Liver cancer
Surgical resection, liver transplant, and tumour ablation therapies are potentially curative treatments for patients with early-stage HCC. However, most patients have advanced disease diagnosis, making them ineligible for curative surgical options [2, 3]. For advanced HCC, transarterial chemoembolisation (TACE) is the standard palliative therapy, delivering chemotherapy directly into the tumour while blocking blood supply [22].
Systemic therapies like sorafenib, an oral multiple tyrosine kinase inhibitor, demonstrate survival benefits in metastatic HCC. Immunotherapies such as checkpoint inhibitors have also shown promising efficacy in clinical trials [25]. Continued integration of newer systemic therapies will be important particularly for advanced stage patients. But improving access to viral hepatitis prevention, screening, and curative therapies for early-stage disease remains the ultimate priority for reducing liver cancer mortality at the global population level [32].
Oesophageal cancer
Oesophageal cancer prognosis depends heavily on the disease stage. While 5-year survival for localised oesophageal cancer approaches 45%, metastatic disease has a dismal 5% survival rate (Table I) [31, 33]. Surgery alone or combined with neoadjuvant chemoradiation provides the best chance for cure in localised oesophageal cancer. Definitive chemoradiation is an alternative treatment option for early-stage disease. Systemic chemotherapy regimens based on fluoropyrimidines and platinums make up the foundation of medical therapy for advanced oesophageal cancer, improving survival and quality of life, versus best supportive care alone.
Even in countries with high treatment capacity like the United States, only about 20% of patients present with potentially operable localised disease. This highlights the critical need to shift oesophageal cancer diagnosis earlier through screening and prompt evaluation of symptoms like dysphagia. Additionally, access to multimodality oesophageal cancer treatment including surgery, systemic therapy, and radiation must expand globally to maintain ongoing improvements in survival (Tables II–IV) [27].
Pancreatic cancer
Pancreatic cancer has an extremely poor prognosis, with 5-year survival under 10% in most countries. Surgical resection provides the only chance for cure, but only about 20% of patients have operable disease at diagnosis (Table I) [29]. Adjuvant chemotherapy is routinely administered after surgery. For inoperable locally advanced or metastatic disease, first-line chemotherapy with gemcitabine and/or multi-agent regimens like FOLFIRINOX can provide survival benefit and symptomatic improvement [1–4].
Newer regimens combining gemcitabine, nab-paclitaxel, and/or targeted therapies have also demonstrated efficacy in clinical trials [29]. The future integration of immunotherapies, oncolytic viruses, and other novel therapies may further advance metastatic pancreatic cancer treatment. But earlier detection through screening high-risk groups offers perhaps the greatest opportunity to improve pancreatic cancer survival on a global population level [22–27].
Regional treatment disparities
Substantial inter-regional variability exists in access to optimal GI cancer treatments and consequently cancer survival. Surgery, chemotherapy, and radiotherapy capacity in Africa, South-Central Asia, and other lower-resourced regions falls dramatically below that in high-income countries (Tables I–IV) [27–33]. For example, fewer than 5% of patients receive adjuvant chemotherapy for gastric cancer in many parts of Africa. Access to newer targeted agents and clinical trial enrolment is also limited. These treatment limitations coupled with higher rates of advanced stage presentation lead to worse GI cancer outcomes in these settings [29].
Closing the gaps in surgical services, chemotherapy availability, radiation infrastructure, cancer surveillance systems, and oncologic expertise through increased healthcare and research investment is key to improving GI cancer survival in disadvantaged world regions. Regional collaborations can also help facilitate training and sharing of services/resources. Ultimately, a global commitment to equitable GI cancer care will be required to end worldwide outcome disparities.
Conclusion on management
Substantial progress has been made in GI cancer treatment, contributing to declining mortality rates for certain cancers in some high-resource countries. However, the majority of patients still succumb to metastatic disease, highlighting the need for both improved treatments and earlier detection [1–11]. Increasing access to multimodal therapy including surgery, systemic therapy, and radiation can significantly improve GI cancer survival, especially in lower-resourced regions facing treatment limitations. Optimal cancer care requires both widespread primary prevention and early detection coupled with universally available state-of-the-art treatments tailored to each individual patient [22–29]. A global commitment to building healthcare infrastructure and ending cancer disparities is essential to reducing GI cancer burden worldwide.
Research priorities
Maintaining the declines in GI cancer incidence and mortality will require ongoing research across the spectrum from prevention to treatment (Table I). Several priority areas stand out as knowledge gaps limiting current ability to curb worldwide GI cancer burden.
Aetiology
While various environmental and lifestyle risk factors for common GI cancers are established, full understanding of susceptibility and carcinogenic mechanisms remains incomplete [1–9]. Further elucidating interactions between genetic, epigenetic, infectious, dietary, metabolic, and other etiologic factors can help refine prevention strategies. Research on GI microbiome changes in carcinogenesis may offer new opportunities for risk modification and early detection.
Vaccines
Prophylactic vaccines against oncogenic infections like H. pylori, hepatitis B and C virus, and human papillomavirus could substantially reduce digestive cancer burden. H. pylori vaccine development is challenged by genetic heterogeneity in strains but remains a key research priority especially for high-stomach cancer regions. Therapeutic cancer vaccines that trigger anti-tumour immune responses are also under study but remain experimental to date [1–7, 9–17].
Screening
While population-based CRC screening is widely implemented, optimal non-invasive, cost-effective approaches with high compliance warrant further study [1–3]. GCR risk stratification to guide tailored screening recommendations is also an active research area. Additionally, ongoing studies are needed to establish best practices for stomach, oesophageal, and liver cancer screening in high-risk groups. Development and validation of effective early detection strategies is key to reducing GI cancer mortality (Tables I–IV).
Treatment
Harnessing the power of precision oncology through molecular profiling of tumours has begun to transform GI cancer therapy [4]. But more work is needed to increase therapeutic targets and match novel treatments like immunotherapy to the patients most likely to benefit. Discovering ways to overcome treatment resistance can also improve outcomes. Additionally, comparative effectiveness research on optimal treatment protocols will help refine evidence-based clinical management across diverse settings [32, 34].
Disparities
Eliminating gaps in cancer prevention, screening, diagnosis, treatment, and survivorship support between advantaged and disadvantaged groups within nations and between geographic world regions requires ongoing health disparities research [9]. A better understanding of barriers across the cancer care continuum can facilitate interventions toward equitable cancer control resource allocation [4].
To propel progress, international collaboration and data sharing coupled with sustained research funding will be imperative. Ongoing discovery across the spectrum of prevention, early detection and management provides the foundation for reducing the global burden of GI cancers [25]. Continued declines in incidence and mortality are achievable through dedicated efforts to generate and apply knowledge that can overcome these highly prevalent and lethal malignancies.
Study limitation
The current review on 5 major GI cancer burdens has some limitation based on exclusive criteria discussed below.
The knowledge base may not capture the latest research developments or statistical trends beyond that point.
As a broad overview, the review cannot provide an exhaustive level of detail on every aspect of epidemiology, prevention, treatment, etc. for each individual GI cancer. More focused literature reviews would be needed to comprehensively cover each cancer site.
The review does not critically evaluate the strengths and weaknesses of the evidence underlying the various preventive strategies, screening methods, and treatment approaches discussed.
The suggestions for research priorities are not prioritised or coupled with a framework for implementation, which could limit their practical impact.
The perspectives presented are primarily from a global standpoint, so the nuances of regional variation and local contexts may not be fully captured.
The review does not quantify the economic burden or cost-effectiveness of the interventions proposed, which could influence feasibility of implementation.
Overall, explicitly acknowledging scope limitations, data constraints, evidence gaps, and the broad geographic perspective would provide appropriate framing for this high-level global review on the burden of GI cancers.
Future prospects and directions in gastrointestinal cancer research and control
The outlook for curbing the burden of GI cancers worldwide is cautiously optimistic. The increasing understanding of GI cancer biology and aetiology coupled with advances in prevention, screening, diagnosis, and treatment provide hope for stepwise reductions in incidence and mortality from these highly prevalent malignancies. However, achieving substantial progress will require global collaboration and commitment from governments, researchers, healthcare providers, and the general public. Increased investment in cancer control infrastructure and human resources, especially in less developed regions, is imperative. According to the literature, further investigation has been conducted into the potential clinical practice implementation of deep learning algorithms for the classification and diagnosis of CRC histopathology images. The advancement made possible by deep learning algorithms has the potential to improve CRC detection’s accuracy and efficacy [35, 36]. Optimisation and broader implementation of vaccination, screening protocols, public awareness campaigns, and multimodal care tailored to local contexts can gradually turn the tide against GI cancers. Continued research across the spectrum from primary prevention to survivorship care will reveal new directions and strategies. The ultimate goal remains lessening the worldwide toll of GI malignancies through knowledge generation, knowledge sharing, and equitable access to evidence-based interventions for all populations.
Discussion
Gastrointestinal cancers pose a major public health threat worldwide as a leading source of cancer incidence, mortality, and disability. Over 5 million people (about twice the population of Mississippi) develop GI malignancies and over 3 million die from these diseases each year globally [1]. Colorectal, stomach, liver, oesophageal, and pancreatic cancers account for the vast majority of the burden. Marked geographic variations exist, with the highest incidence rates found in developed countries like Australia, New Zealand, Europe, and North America. Mortality is highest in less developed regions like Asia and Africa [2].
These patterns reflect differences in risk factor profiles and cancer prevention and control capacities between populations [3]. However, GI cancer incidence is rising across traditionally low-risk countries concurrently with economic development and adoption of unfavourable lifestyle changes like smoking, poor diet, and physical inactivity (Table II) [4]. The increasing burden coupled with frequently poor prognosis highlights the urgent need for expanded prevention efforts and research focus on GI cancers.
In the last few years, technological developments in the surgical field have been rapid and are continuously evolving. One of the most revolutionising breakthroughs was the introduction of the IoT concept within the surgical practice [37]. The Internet of Things (IoT) has the potential to play a significant role in GI cancer screening and early detection. IoT technology can facilitate more convenient, accessible, and efficient GI cancer screening processes. Wearable biosensors and ingestible devices can continuously monitor indicators like biomarkers, gut microbiome changes, or GI symptoms that may signal early cancer development. This data can be seamlessly transmitted to healthcare providers via internet connectivity, allowing for remote monitoring and early intervention when abnormalities are detected. IoT-enabled smart toilets can analyse stool samples and alert users/physicians about the presence of blood or other potential cancer signs. Moreover, IoT systems can improve screening program adherence by sending automated reminders to patients about upcoming screening appointments or home-based testing kit schedules. The integration of IoT with AI and machine learning can further enhance the accuracy and personalisation of cancer risk prediction models, enabling tailored screening recommendations based on individual risk profiles. However, issues around data privacy, cybersecurity, and regulatory oversight need to be carefully addressed for the safe and effective implementation of IoT in GI cancer screening programs [37].
Key strategies to curb the GI cancer epidemic include rolling out vaccination for infections that elevate cancer risk, reducing environmental carcinogens, promoting lifestyle changes to decrease obesity and alcohol abuse, expanding screening programs, and improving access to optimal cancer treatments globally [5, 6]. However, achieving substantial reductions in GI cancer incidence and mortality will require coordinated, multilevel efforts. Governments must prioritise cancer control in health policy and dedicate adequate resources. The research community needs to continue elucidating GI carcinogenesis and identifying optimal prevention and treatment approaches. Healthcare systems should implement widespread screening programs and provide team-based, patient-centred cancer care. Public health practitioners must disseminate cancer prevention messaging and work to reduce disparities. A collaborative commitment from all stakeholders is necessary to eventually overcome the global burden of GI cancers.
Conclusions
GI cancers collectively represent a substantial global public health burden, resulting in over 3 million deaths annually. Colorectal, stomach, liver, oesophageal, and pancreatic cancer account for the vast majority of GI cancer cases and deaths worldwide. Marked geographic variations exist, with the highest incidence rates typically seen in developed countries like Australia/New Zealand, Europe, and North America and the highest mortality in less developed regions like Asia and Africa.
These patterns reflect differences in risk factor profiles and cancer prevention/control capacities between populations. However, incidence is rising across traditionally low-risk countries concurrently with economic development and adoption of unfavourable lifestyle changes. The increasing global burden coupled with poor prognosis for many GI malignancies highlights the urgent need for expanded prevention efforts and research focus.
Key priorities include implementation of widespread vaccination against oncogenic infections, reducing environmental carcinogens, and promoting lifestyle changes to curb obesity, smoking, unhealthy diets, and alcohol overuse (Table III). Secondary prevention through earlier detection and treatment of pre-malignant lesions and localised cancers via screening and improved access to optimal surgery, chemotherapy, and radiation will also be critical, especially in lower-resource regions. Continued research on prevention, screening, treatment advances, disparity reduction, and all aspects of GI cancer control will provide the pathway to eventually overcome these major causes of global cancer burden.
These patterns reflect stark differences in risk factor profiles and cancer control capacities globally. However, GI cancer burden is rising across traditionally low-risk countries along with economic development and adoption of unhealthy lifestyle behaviours like smoking, poor diet, and physical inactivity. The increasing incidence coupled with frequently poor prognosis highlights the urgent need to curb the GI cancer epidemic through expanded prevention efforts, early detection, treatment access, and research prioritisation.
Strategies such as vaccination for oncogenic infections, reducing environmental carcinogens, promoting lifestyle changes, implementing screening programs, and improving multimodal cancer treatment availability could substantially reduce GI cancer burden if applied universally. However, achieving significant declines in GI cancer morbidity and mortality will require coordinated global actions across the cancer control continuum, from primary prevention to palliative care. Governments must prioritise cancer control in health policy and resource allocation. Healthcare systems need to provide cost-effective, equitable screening and care. Researchers should continue elucidating GI cancer biology and identifying optimal prevention and treatment approaches. Public health practitioners must disseminate cancer prevention messaging and work to reduce disparities.
Collaborative commitments from policymakers, researchers, providers, and the general public will be essential to conquer the substantial worldwide toll of GI cancers. The outlook for curbing the GI cancer burden is cautiously optimistic if evidence-based interventions can be broadly and equitably implemented through global cooperation and resolve.
Overall, further research on GI cancer strategy in the gut may enable translation of its physiological functions into therapeutic strategies for maintaining intestinal health and restoring homeostasis in functional and GI cancers.