Introduction
Autoimmune hepatitis (AIH) is the prototype autoimmune liver disease both in adults and children, having been the first to be described in the 1950s. It is a progressive inflammatory hepatopathy, which, if untreated, evolves to end-stage liver disease. The most typical features of AIH are female preponderance, hypergammaglobulinemia/increased immunoglobulin (Ig) G, seropositivity for circulating autoantibodies, and a picture of interface hepatitis on histology [1].
Autoimmune hepatitis responds to immunosuppressive treatment in the majority of cases. Treatment should be instituted promptly upon diagnosis. If left untreated, AIH usually progresses to liver failure requiring transplantation [2].
Glucocorticoids (GCs) can effectively relieve AIH, but some patients with this disease are refractory even when GCs are administered. They include patients at increased risk for disease progression and further development of hepatic failure. These patients are in need of more aggressive immune-modulating treatment, and valuable time may be lost awaiting the absent response to standard treatment [3].
The physiological and pharmacological effects of GCs are mediated through the binding to the glucocorticoid receptor (GR), a protein belonging to the superfamily of nuclear hormone receptors (gene ID: NR3C1) [4].
11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) is a crucial enzyme that converts inactive cortisone into active hydrocortisone, thus regulating multiple functions of GCs. 11β-HSD1 is widely expressed in tissues and is particularly abundant in the liver, muscle and fat tissue [5].
Previous studies have investigated the GR and 11β-HSD1 in extra-hepatic tissues [6-8], while studies assessing its expression in liver tissues are scarce. So, our study was performed to investigate the hepatic expression of GR and 11β-HSD1 in pediatric AIH and their relations with the steroid response.
Material and methods
This retrospective cohort hospital-based study was conducted on 100 patients diagnosed with AIH. The patients were recruited from the outpatient clinic and the ward of pediatric hepatology, gastroenterology and nutrition department at the National Liver Institute, Menoufia University, Egypt from 2015 to 2020. Informed consent was obtained from the guardians of the children after obtaining the local ethical committee approval.
The study included children up to 18 years old diagnosed with AIH according to the ESPGHAN Hepatology Committee diagnostic criteria of pediatric AIH [2]. Patients with an associated liver disease such as chronic hepatitis B or C or Wilson disease (identified by either laboratory or histological examination) were excluded from the study.
The data were collected from the files of the patients and included: demographic data, clinical presentation, clinical examination, biochemical parameters including complete blood count (CBC), liver function test, serum autoantibodies, serum IgG concentration, hepatitis B and C viral markers, radiological and histological findings, treatment response and outcome.
The studied patients received immunosuppressive therapy according to the AIH protocol [9]. When patients achieved clinical and biochemical remission for the first time they were followed for a period of 12 months. At the end of this period, patients were classified according to their response to steroid therapy as complete, partial and non-response. Complete response was defined as normalization of liver enzymes and absence of clinical symptoms (normal alanine transaminase [ALT] on a minimum of two occasions at least a month apart). Partial response (incomplete response) was defined as improvement in liver enzymes without normalization. Non-response (treatment failure) was defined as failure of clinical, laboratory, or histological improvement despite compliance with therapy [10].
Liver biopsy and histopathological evaluation
An ultrasonography-guided liver biopsy was performed for all patients during the diagnostic workup using a true-cut needle, size 16G. A biopsy with liver tissue containing 10 or more portal tracts is considered adequate [11]. Biopsy specimens were fixed in formalin, embedded in paraffin and subjected to the following:
Routine histopathological evaluation (staining with hematoxylin and eosin [H&E], Masson’s trichrome, Perls’ Prussian blue stain and Periodic acid-Schiff [PAS] stain). Routine histopathological assessment was based on the evaluation of necroinflammatory changes (grades) and architectural changes (stages) recorded as the histological activity index (HAI) and fibrosis score, respectively. They were performed according to the scoring system of Ishak and colleagues [12].
Evaluation of immunostaining results (11β-hydroxysteroid dehydrogenase type 1 [11β-HSD1] and glucocorticoid receptor [GR] immunostaining using antibodies against 11β-HSD1 and GR).
Immunostaining technique: Four µm-thick sections were cut from a paraffin block, mounted on Super frost Plus slides (Thermo Fisher Scientific, Waltham, MA, USA), deparaffinized in xylene and rehydrated in descending grades of ethyl alcohol. Endogenous peroxidase was blocked using 0.3% hydrogen peroxide in phosphate buffer saline (PBS). After two washes with PBS, sections were incubated with a biotin blocking system (Dako, Glostrup, Denmark) followed by incubation with antigen retrieval solution (Dako, Glostrup, Denmark) for 10 minutes then 5 washes with distilled water. Slides were incubated with the primary antibodies horizontally in a humidity chamber overnight, at room temperature. After several washes in PBS, the slides were incubated with biotin-conjugated secondary antibody using goat anti-mouse anti-rabbit immunoglobulin (Dako, Glostrup, Denmark) and then incubated with streptavidin-horseradish peroxidase (HRP). The reaction was visualized using diaminobenzidine (DAB) followed by hematoxylin counterstaining. (All were done using LSAB2 System-HRP, Dako, Glostrup, Denmark.) Immunostained sections were evaluated by a liver histopathologist.
Localization of 11β-HSD1 antibody was determined even if in the cytoplasm of hepatocytes, bile duct epithelium or inflammatory cells [13]. The following values were assigned: “0” – no immunostaining, “1” – weak immunolabelling, “2” – moderate immunolabelling, and “3” – strong immunostaining [14].
Localization of GR antibody was assessed as to whether it was in the nucleus and cytoplasm of hepatocytes, bile duct epithelium or in the endothelial cells lining blood vessels [15]. Glucocorticoid receptor reactivity was scored using differential intensity scores (0 – null, 1+ – low or weak, 2+ – moderate, 3+ – high or strong). A total percentage score (% of cells staining ≥ 1+ intensity; i.e., the sum of the percentage of cells at 1+, 2+, and 3+ intensities) was used to semi-quantitatively evaluate cell expression of GR [16].
Statistical analysis
Data were collected and analyzed using the program SPSS Statistics, version 18 (SPSS Inc., Chicago). Data were entered as numerical or categorical data, as appropriate. Quantitative data were shown as mean ± standard deviation (SD) (minimum-maximum) or median (25th-75th percentile) as appropriate. Qualitative data were expressed as frequency and percent (%). The chi-square test and Fisher’s exact test were used to assess the association between qualitative variables. The Kruskal-Wallis test was conducted to compare medians of more than 2 sets of quantitative data and the Mann-Whitney U test was used to compare medians of 2 sets of quantitative data non-parametrically distributed. The analysis of variance (ANOVA) test was performed to compare means of more than 2 sets of quantitative data parametrically distributed. The paired sample t-test and Wilcoxon signed ranks test were used to assess the follow-up of 2 sets of quantitative parametric and non-parametric data, respectively. Spearman correlation was used to assess the correlation between quantitative variables. The p (probability) value was considered to be statistically significant if it was ≤ 0.05.
Results
The study included 100 patients with AIH on immunosuppressive therapy; 82 cases showed a complete response, 11 a partial response and 7 cases were nonresponders. The sex and age distribution and clinical presentation of the disease were comparable among the different response groups (p > 0.05) (Table 1).
Table 1
Sex, age distribution and clinical presentations among the different response groups
The laboratory parameters before the beginning of treatment in the different studied groups showed no statistically significant difference except for γ-glutamyl transferase (GGT), which was lower in patients with a complete response than in partial responders (p = 0.017). In addition, ASMA positivity increased significantly in complete and partial responders (p = 0.038). In ultrasound, hepatomegaly, splenomegaly and ascites were not predictors of a response to therapy (p > 0.05) (Table 2).
Table 2
Laboratory parameters and ultrasound findings of autoimmune hepatitis (AIH) patients according to response to steroid before treatment
[i] ALT – alanine transaminase, ALP – alkaline phosphatase, ANA – antinuclear antibodies, anti-LKM – anti-liver-kidney microsomal antibodies, ASMA – anti-smooth muscle antibodies, AST – aspartate transaminase, GGT – χ-glutamyl transferase, INR – international normalized ratio, WBC – white blood cells
Type 1 AIH represented 73.2%, 90.9% and 71.4% of complete, partial and non-responder groups, respectively, while seronegative AIH represented 15.9%, 9.1% and 28.6% of complete, partial and non-responder groups, respectively. Type 2 AIH was present in the complete response group only (11%), with no significant difference between the different response groups as regard the types of AIH (p > 0.05). The mean AIH score was comparable between the 3 response group (p > 0.05), 14.8 ±2.81 in complete response, 14.5 ±2.25 in partial response and 14 ±2.89 in non-response groups. In addition, the treatment lines steroid only regimen and combined regimen were not predictors of a response to therapy (p > 0.05).
The histopathological findings were comparable between the different response groups (p > 0.05) (Table 3). Immunohistochemical staining of 11β-HSD and GR in liver tissue showed different intensities within the different hepatic tissues (Figures 1 and 2). Glucocorticoid receptor reactivity was significantly more intense in patients with a complete response than both patients with a partial response and non-responders. 11β-HSD intensity was higher in complete and partial responders in comparison with non-responders but without significance. The percentage of patients with a GR intensity score ≥ 200 was significantly higher in patients with a complete response than patients with a partial response and non-responders (p < 0.05) (Table 4). The GR intensity score had a significant positive correlation with intensity of 11β-HSD (r = 0.369, p < 0.0001).
Table 3
Histopathological findings among autoimmune hepatitis (AIH) patients according to response to steroid
Table 4
11βl-hydroxysteroid dehydrogenase (11βl-HSD) and glucocorticoid receptor (GR) intensity scores among autoimmune hepatitis (AIH) patients according to response to steroid
Fig. 1
Autoimmune hepatitis (AIH) showed cytoplasmic expression of glucocorticoid receptor (GR). A) A case of AIH showed cytoplasmic expression of GR within parenchymal hepatocytes (yellow arrow) and endothelial (red arrow) (integrated professional studies, 200×). B) A case of AIH showed cytoplasmic expression of GR within portal inflammatory infiltrate and bile ductules (red arrows) (200×)
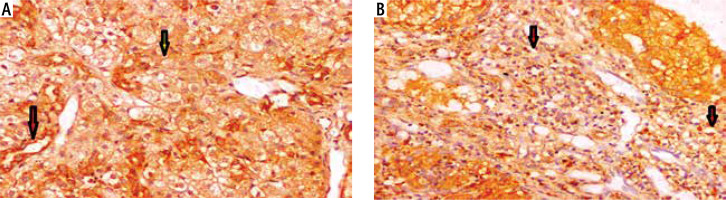
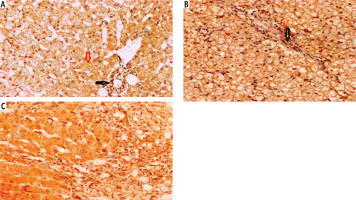
Fig. 2
Autoimmune hepatitis (AIH) cases showed different intensities of cytoplasmic expression of 11β-HSD. A) Score 1 within hepatocytes (yellow arrow), bile duct epithelium, and inflammatory cells in portal tracts (black arrow) (integrated professional studies, 200×). B) A case of AIH showed moderate cytoplasmic expression (score 2) of HSD1 within hepatocytes, bile duct epithelium (yellow arrow), and inflammatory cells in portal tracts (200×). C) Score 3 of HSD1 within hepatocytes, bile duct epithelium, and inflammatory cells in portal tracts (200×)
On comparing the 11β-HSD and GR intensity score in males and females, both 11β-HSD and GR were significantly more intense in females. Median (25th-75th percentile) of 11β-HSD was 2 (1-3) in females vs. 1 (1-2) in males (p < 0.015). Median (25th-75th percentile) of GR intensity score in females was 200 (100-300) vs. 100 (100-200) in males (p < 0.031).
The GR and 11β-HSD expression showed no statistically significant difference among different stages of fibrosis or different grades of necroinflammatory activity (p > 0.05) (Table 5).
Table 5
Glucocorticoid receptor (GR) and 11β-hydroxysteroid dehydrogenase (11β-HSD) expression among different stages of fibrosis and different grades of necroinflammatory activity
Discussion
In juvenile autoimmune hepatitis, treatment is associated, in over 90% of cases, with a measurable clinical and laboratory response within 4 to 8 weeks. Complete normalization of biochemical parameters may, however, take several months [17].
In the present study, most of the cases received combined therapy (steroid + azathioprine) as standard treatment. Conventional treatment was associated with a measurable clinical and laboratory improvement within 8 weeks in 82% of our patients. An incomplete response and no response were present in just 11% and 7% of our patients, respectively. This is in accordance with a study by Sheiko et al., which reported a remission rate of about 90% using steroid ± azathioprine [18]. In addition, Malik and Venkatesh reported that an incomplete response occurred in 14% of their patients and treatment failure occurred in 7% [19].
On analyzing the probable predictors for a response to steroid therapy, patient gender, age, clinical presentation, laboratory parameters and AIH score were not found to be predictors of a response to steroid therapy except for GGT, which had lower levels in the complete response group. Concomitant with our finding, Behairy et al. found that the median GGT level was significantly higher in the non-responder group [20].
Liver biopsy is an essential tool in the diagnosis of AIH. In our study, the histopathological findings regarding fibrosis stage, activity grade, portal inflammation, AIH grade, spotty necrosis, and confluent necrosis were quite variable among patients, yet none of these findings were predictors of a response to therapy.
Interestingly, the GR expression showed great variability in the different response groups, with significantly more intense expression in patients with a complete response followed by partial responders and the lowest expression was in non-responders. Quinn et al. stated that GC sensitivity can be modulated via multiple mechanisms, particularly by altering the expression of GR. They hypothesized that the hepatic GC resistance is due to the reduction in receptor expression [21]. In addition, Eriksen et al. explained the failure of the standard treatment regimen of prednisolone and azathioprine among AIH patients due to presence of variants in genes related to GR signaling. They suggested that variations in these genes resulted in variation in GR expression with different subsequent responses to steroid therapy [22].
These predefined genetic and histopathological factors may offer part of the pathophysiological explanation for difficult to treat AIH cases and should be further explored. One perspective is the potential to immediately identify patients who need non-standard immune therapy to obtain rapid disease control in the hope of preventing some of the dangerous clinical courses of AIH and unwanted side effects of steroids.
The actions of glucocorticoids on target tissues are not necessarily dependent on circulating glucocorticoid levels but rather on their pre-receptor metabolism. Glucocorticoid action on target tissues is determined by the density of “nuclear” receptors and intracellular metabolism by the isozymes of 11β-HSD which catalyze interconversion of active cortisol and corticosterone with inert cortisone and 11-dehydrocorticosterone. The predominant sites of 11β-HSD1 action are liver, muscle and adipose tissue. In these tissues, 11β-HSD1 acts predominantly as a reductase to regenerate active glucocorticoids and thereby regulates access of glucocorticoids to the GR. Activation of 11β-HSD1 and GR could result in the production of excess tissue glucocorticoids and induction of GR-mediated local glucocorticoid action [23].
Although 11β-HSD1 activity has been shown to play an important role in the metabolic actions of glucocorticoids, its role in the immune response still remained unclear. We hypothesize that the regulation of cortisol by 11β-HSD1 in liver tissue is a determinant of the pathogenesis of AIH and modulation of this enzyme may provide a novel therapeutic target for treatment of this common sight-threatening disorder.
Unfortunately, on assessing the expression of 11β-HSD1 in liver tissues of patients with AIH, we did not find a significant difference of 11β-HSD1 expression between different response groups. However, we observed that 11β-HSD1 expression was higher in complete and partial responders than non-responders. In addition, the expression of 11β-HSD had a significant positive correlation with the GR intensity score.
Although no previous studies have assessed 11β-HSD1 expression in AIH disease, several studies have assessed the hepatic 11β-HSD1 expression in other liver diseases, with conflicting results. For example, Konopelska et al. reported that there was no association between hepatic 11β-HSD1 expression and the pathology of fatty liver or non-alcoholic steatohepatitis (NASH) in humans [24], whereas(Ahmed et al. suggested that in the early stages of nonalcoholic fatty liver disease (NAFLD), with steatosis alone, hepatic 11β-HSD1 activity decreases with the progression to NASH, which is associated with increased 11β-HSD1 levels [25]. Lutz et al. found that genetic variation in 11β-HSD1 was associated with visceral and particularly with liver fat accumulation but not substantially with whole-body insulin resistance [26]. These data indicate that chronic inhibition of 11β-HSD1 may be a valuable approach to treat NAFLD and visceral obesity.
On the other hand, in the present study, the hepatic expression of 11β-HSD1 and GR intensity showed no correlation with the stages of liver fibrosis or the grades of necroinflammation, although Zou et al. found that specific deletion or inhibition of 11β-HSD1 enhanced the activation of HSCs in a murine model of liver fibrosis [27].
In conclusion, GR expression in liver tissues was a key in the response of the AIH patients to steroid therapy. Immunostaining for GR reactivity in liver tissue should be routine histochemical staining for proven cases of AIH before beginning the treatment regimen.






