Introduction
These guidelines are an update of the 2013 diagnostic and therapeutic recommendations of the Polish National Consultant in Gastroenterology and the Polish Society of Gastroenterology (PTG-E) for the management of adult patients with ulcerative colitis (UC), as amended [1]. The update was prepared by the Inflammatory Bowel Disease (IBD) Working Group of PTG-E.
Objective
The main objective of this document is to complete the guidelines already in force to include new information, particularly regarding new medicines which have been approved for use in UC since 2013 as well as to popularise and unify therapeutic algorithms in UC. As a consequence, the overriding objective of the guidelines is to provide patients in Poland with better access to modern diagnostic tools and treatment of UC, based on the current state of knowledge and evidence. At the same time, the previous guidelines were reviewed in terms of substance as well as methodology according to most of the current recommendations of the Agency for Health Technology Assessment and Tariff System (AOTMIT) on the principles for the construction of guidelines [2].
Health problems addressed in the guidelines
The recommendations address the following issues in detail:
the epidemiology of UC, including the dynamics of incidence and morbidity trends in recent years,
the symptoms and clinical presentations of UC,
the diagnostic approaches in patients with suspected UC (including differential diagnosis) and UC diagnostic criteria,
current recommendations for therapeutic management of UC, including pharmacological and surgical treatment as well as psychological support, and the principles of coordinated, holistic care for UC patients.
Target patient population
These recommendations are for the management of adult patients (over 18 years of age) with suspected or confirmed diagnosis of UC, regardless of the disease phenotype and the severity of symptoms.
Epidemiology and general characteristics of UC
UC is a chronic incurable inflammatory disease of the large intestine with an unknown cause, in which the inflammatory process involves mainly the mucosa and is limited to the rectum or rectum and colon [3]. The most common clinical symptom of UC is diarrhoea with the presence of rectal bleeding. Additionally, abdominal pain, weight loss, subfebrile state/fever, and extraintestinal symptoms may be present. Occasionally – in patients with rectal involvement only – constipation may occur [3].
UC is a disease of young people; it usually begins in the second or third decade of life, but in recent years there has been a trend of increasing incidence of UC in older people, including those over 60–65 years of age. The annual incidence in Europe is estimated at around 10 new cases per 100,000 people [3, 4]. As indicated by Polish data, in 2018 the UC incidence (number of new diagnoses) standardised for the age of the European population was 12.3 per 100,000, and UC prevalence (the number of people living with the disease and newly diagnosed) was 187.8 per 100,000 (in 2020, about 74,000 people lived with UC) [5].
Definitions [1, 6, 7]
Active disease – it is diagnosed when the patient experiences clinical signs and symptoms accompanied by the presence of measurable inflammatory markers (biochemical markers such as elevated faecal calprotectin level, endoscopic and/or microscopic signs of inflammation).
Clinical remission – the absence of signs and symptoms of active disease. Usually, clinical remission is considered to be up to 3 bowel movements per day without signs of lower gastrointestinal bleeding. Several scales may be used to assess the clinical condition of a patient with UC (discussed in Recommendation No. 2). The patient is considered to be in clinical remission when the PRO-2 (patient reported outcomes-2) score is 0 (see Recommendation No. 2).
Endoscopic remission – the absence of inflammatory activity in endoscopic examination. The Mayo Score is most commonly used to assess the severity of endoscopic lesions. It is part of the Total Mayo Score and is presented in Recommendation No. 2. Endoscopic remission is considered to be a score of 0 on this four-point scale.
Clinical response – an improvement of the patient’s general clinical condition, understood as a significant reduction of symptom severity. It is sometimes defined as a reduction of the PRO-2 score by at least 50%.
Endoscopic response – an improvement in the endoscopic appearance from the initial assessment. It is sometimes defined as a reduction in the Mayo score of disease activity by at least 1 point.
Relapse – reappearance of active disease in a patient who has been in remission. An early exacerbation is considered to occur within 3 months of achieving remission.
Extensive UC – it refers to a situation in which inflammatory lesions involve the large intestine proximal to the splenic flexure (and thus are present in at least the rectum, sigmoid colon, descending colon and left part of the transverse colon). The Montreal classification of UC defining disease phenotypes according to the disease extension is presented in Table I.
Table I
Goals of treatment – treatment of UC consists of the remission induction phase aimed at improving the disease course in patients with exacerbation, and of the maintenance phase aimed at maintaining the improvement achieved as a result of induction therapy and reducing the risk of subsequent relapse.
In the short term, the goal of therapy is to achieve a clinical response confirmed by objective methods (endoscopic or biochemical – mainly through the assessment of faecal calprotectin levels). The main goal of UC treatment is to obtain sustained resolution of all disease symptoms (full clinical remission) along with normalisation of the endoscopic findings (endoscopic remission), and thus to restore the patient’s chance for a normal unrestricted personal, social and professional life.
Steroid-refractory disease – a clinical situation where during disease exacerbation a remission cannot be obtained despite the use of steroids at the full dose for 4 weeks. In patients with acute severe ulcerative colitis (ASUC), steroid-refractory disease is defined as no response after 3 days of intravenous steroid therapy.
Steroid-dependent disease – impossibility to reduce the steroid dose below an equivalent of 10 mg of prednisone or 3 mg of budesonide per day within 3 months of treatment or exacerbation within 3 months after steroid therapy termination.
Primary nonresponse – the lack of clinical improvement after the completion of induction treatment. Like the definition of loss of response, this definition is most often used in the context of biological treatment.
Loss of response – relapse in the course of maintenance treatment in a patient in whom clinical remission was previously achieved. This definition is often extended to include patients in whom the dose of the medicine used for maintenance treatment had to be increased in order to maintain the remission.
Guideline development methodology
These guidelines were drawn up by a group of experts appointed by PTG-E and the Polish National Consultant in Gastroenterology. The group initiated the development of the guidelines by formulating the preliminary principles and a list of clinical issues and problems based on the recommendations already in force, which were then updated in line with current knowledge according to the PICO (Patients, Intervention, Comparator, Outcome) protocol [8, 9]. Major updates were required as to the place of novel medicines in the UC treatment algorithms.
At all stages of the drafting, recommendations were developed on the basis of source data identified from the search of electronic databases (PubMed, Cochrane Library, and Embase) as well as guidelines published by international scientific societies, i.e. European Crohn’s and Colitis Organisation (ECCO), American Gastroenterological Association (AGA), American College of Gastroenterology (ACG) and British Society of Gastroenterology (BSG), with particular consideration of documents based on the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) methodology [3, 6, 9–13].
Quality and strength of the available therapeutic recommendations were assessed using a GRADE-based methodology. For each recommendation, the quality of the evidence (Table II: high, moderate, low, very low) and the strength of the recommendation (Table III: strong and weak recommendation) were determined by the experts [14].
Table II
Table III
Criteria for assessing the strength of recommendations [14]
After the recommendations were formulated and their strength and quality of evidence were assessed, the final version of each recommendation was analysed in detail. The degree of experts’ approval of the proposed final phrasing of the recommendation, its strength and the quality of supporting evidence were assessed on a 6-point Likert scale, with 1 corresponding to complete disapproval/lack of support, 2 corresponding to disapproval/lack of support, 3 corresponding to partial disapproval/lack of support, 4 corresponding to partial approval/support, 5 corresponding to approval/support, and 6 corresponding to complete approval/support (Table IV) [15].
Table IV
The Likert scale [15]
| Approval rating according to the Likert scale | |
|---|---|
| 1 | Complete disapproval |
| 2 | Disapproval |
| 3 | Partial disapproval |
| 4 | Partial approval |
| 5 | Approval |
| 6 | Complete approval |
Recommendations could be revised after voting. If > 75% of the panellists rated support for a given recommendation on the Likert scale at 4–6 points (high consensus rate), the recommendation was considered finally accepted. The consensus of ≤ 75% was considered low [15].
The next step involved assessment of the quality of the guidelines using the AGREE II tool pursuant to the AOTMIT guidelines available at www.aotm.gov.pl. All comments were included in the final version of the recommendations [2].
Interpretation of the guidelines
Each therapeutic recommendation is accompanied by the following three pieces of information:
I. Diagnostic evaluation
1. The diagnosis of ulcerative colitis is based on clinical assessment and endoscopic evaluation of the large intestine with histopathological analysis of colonic biopsies. At the same time, other disease entities with similar symptomatology (mainly infectious diseases) should be excluded. Biochemical tests and radiological examinations are important complements to the diagnostic process.
| Recommendation #1 – approval rating (Likert scale) | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
The most common clinical symptom of UC is diarrhoea with the presence of lower gastrointestinal bleeding [1, 3, 12]. It may be accompanied by abdominal pain, weight loss, subfebrile state/fever, and sometimes extraintestinal manifestations may be present. Occasionally – in patients with rectal involvement only – constipation may occur. Endoscopic assessment of the large intestine is essential in making the right diagnosis. In patients with a severe clinical form of the disease, the examination of choice is rectosigmoidoscopy performed without any prior bowel preparation. In any other case, the primary endoscopic examination is ileocolonoscopy with macroscopic evaluation and collection of at least two biopsy specimens for histological examination from all inspected segments of the intestine [1, 3, 6]. Inflammatory lesions in UC are usually continuous and limited to the rectum or to the rectum and colon. These usually include loss of the vascular pattern, erythema, granularity, spontaneous or contact bleeding of the mucosa, and the presence of erosions or flat, or sometimes deep, ulcers. A sharp demarcation between the inflamed and normal mucosa is a characteristic sign. The microscopic signs suggestive of UC, but not its hallmark signs, include intestinal crypt architectural distortion (such as the presence of irregular branched crypts or atrophic crypts) accompanied by inflammatory infiltration with predominance of lymphoplasmocytes in the area of the epithelial basement membrane and mucosal lamina propria as well as by granulocyte infiltration within the epithelium of intestinal crypts (cryptitis), and the crypt abscesses [1, 3, 6].
An indispensable complement to the diagnostic process is differentiation from other disorders having a similar clinical, endoscopic and histological presentation. In particular, infectious diseases should be ruled out. The necessary diagnostic tests depend on the clinical context. An infection with toxigenic Clostridioides difficile should usually be excluded; sometimes it is necessary to test the patient for an infection with Salmonella/Shigella or Entamoeba histolytica. In the case of an unusual presentation, it is worth testing for human immunodeficiency virus (HIV) infection [1, 3, 6, 12, 16].
Biochemical tests and radiological examinations have a complementary role. The faecal calprotectin level well correlates with the severity of inflammation in the large intestine. It is believed that the value > 250 µg/g of stool indicates significant severity of the inflammatory process, but is not sufficient to make a diagnosis and does not eliminate the need for endoscopy [17, 18]. Among blood tests, it is important to assess total blood cell count, iron metabolism parameters and the C-reactive protein (CRP) level, but the lack of abnormal results does not rule out UC.
The basic radiological examination should be abdominal ultrasound with intestinal assessment, which in many cases makes it possible to non-invasively assess the extent and severity of lesions. Double contrast examination of the large intestine (with a positive contrast, e.g. barium, and negative contrast, i.e. the air) is nowadays only exceptionally performed. It is contraindicated in patients with high activity of UC.
The final diagnosis of UC is made by the clinician on the basis of an analysis of the available clinical data and results of diagnostic tests [17, 18].
2. In addition to clinical examination, endoscopic evaluation plays a pivotal role in the assessment of the activity of ulcerative colitis. Faecal calprotectin is the most reliable biochemical parameter reflecting inflammatory activity of the disease. The need for other recommended biochemical tests should be determined on a case-by-case basis.
| Recommendation #2 – approval rating (Likert scale) | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 8% | 8% | 84% | |||
Every patient diagnosed with UC requires medical supervision. Scales that take into account the most important clinical and endoscopic parameters of the severity of pathological changes may be helpful in the assessment of disease activity. One of the most commonly used scales is the Total Mayo Score (Table V) [1, 3, 7, 19].
Table V
Total Mayo Score (TMS) assessing the activity of ulcerative colitis [1]
Interpretation: 0–2 points (and all individual variables < 2 points) – remission, 3–5 points – mild activity, 6–10 points – moderate activity, > 10 points – severe activity.
* The Partial Mayo Score includes the assessment of all components except for the endoscopic subscore. The Modified Mayo Score includes all components except for the physical rating of disease activity [19].
In recent years, particular attention has been paid to patient reported outcomes (PRO), among which the following components of the Total Mayo Score are of key importance: the stool frequency above normal per day and rectal bleeding (the PRO-2 scale) [7].
The faecal calprotectin level well reflects the severity of inflammation in the large intestine; therefore it should be routinely measured in the surveillance of patients in remission and in suspected exacerbation of UC. The cut-off point below which mucosal healing should be expected is usually considered to be 150 µg/g of stool [17]. Other laboratory tests (e.g. complete blood cell count, iron metabolism parameters, CRP, albumin) are a valuable complement to the diagnostic evaluation and have an auxiliary role. The most useful radiological examination is the assessment of UC activity in ultrasound examination of the intestines [1, 18].
3. In a patient with exacerbation of ulcerative colitis it is necessary to rule out the coexistence of an underlying infection contributing to the symptoms.
| Recommendation #3 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
Patients with IBD are at increased risk of infectious diseases. Therefore, the worsening of symptoms in a patient diagnosed with UC may result from the overlap of an infectious disease [6, 16]. The scope of diagnostic tests differentiating the cause of diarrhoea and possibly blood in the stool depends on the clinical situation, but it is usually necessary to rule out an infection with toxigenic Clostridioides difficile. Sometimes it is recommended to perform tests for an infection with such micro-organisms as Shigella/Salmonella, E. histolytica or pathogenic types of Escherichia coli. In steroid-refractory disease, diagnostic evaluation for reactivated cytomegalovirus infection should also be considered (proposed method – assessment of immunohistochemical expression of cytomegalovirus in colonic biopsy specimens) [6, 16, 20].
II. Treatment
In general, pharmacological treatment of UC is based on a step-up strategy, i.e. gradual introduction of medicines with an increasing immunosuppressive potency upon failure of the previous treatment methods [3, 9, 12, 13]. To avoid prolonged treatment with an ineffective medicine, upon treatment initiation or modification a time should be set for the assessment of its effects, depending on the medicine used. The step-up strategy does not apply to cases of acute severe UC. In this situation, in view of the risk of systemic complications and the high colectomy rate, intense treatment based on medicines with the greatest therapeutic potential should be initiated as soon as possible [3, 9, 12, 13].
Planning of pharmacological treatment should be personalised and based on at least the following criteria [3, 7, 9, 12, 13]:
Disease activity – determined on the basis of clinical scales (e.g. the Mayo Score, PRO-2, Truelove and Witts criteria) and endoscopic scales (the Mayo Endoscopic Subscore). It is important, among other things, for the selection of first-choice therapy as well as for defining the time of assessing treatment efficacy.
Extent of inflammatory lesions – on the basis of endoscopic or imaging examinations. It is taken into account, for example, when selecting the route of administration of the medicine used.
Disease history – assessment of the efficacy of the existing treatment, the number of exacerbations, the pharmacological therapy that led to a remission in previous exacerbations.
Pharmacological treatment of UC consists of two phases: the induction treatment aimed at obtaining clinical remission, and preferably also endoscopic remission, and subsequently the maintenance treatment aimed at maintaining the remission status without further exacerbations [3, 9, 11–13].
An important element of assessing treatment efficacy is the assessment of the healing of mucosal lesions. It has been evidenced that achieving endoscopic remission is associated with a lower risk of subsequent exacerbations. However, the data on this subject are less conclusive than in the case of Crohn’s disease. This goal is much more difficult to achieve than only clinical remission [3, 7, 9, 11–13].
Mild-to-moderate activity
4. We recommend treatment with mesalazine (administered orally and/or rectally) for mild-to-moderate exacerbations. Combination treatment with an oral and rectal formulation is more effective than treatment with an oral or rectal form alone.
(Quality of evidence: moderate; strength of recommendation: strong)
| Recommendation #4 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 8% | 92% | ||||
Mesalazine (5-aminosalicylic acid) is the drug of first choice for mild-to-moderate exacerbations [21–27]. If the extent of lesions is limited to the rectum (proctitis), treatment should be started with a topical formulation – a suppository at a dose of 1 g/day, usually applied in the evening [3, 9, 11, 21–27]. In patients with rectal and sigmoid involvement, the procedure of choice may be the use of mesalazine in rectal enema at a dose ≥ 1 g/day [3, 9, 11–13, 28–37]. If the lesions are confined to the left half of the colon, a combination treatment with an oral formulation (at least 3 g/day, as a single dose or in divided doses) and a topical formulation (rectal enema or suppository) should be used. If the lesions are extensive (proximal to the splenic flexure), an oral formulation (at a dose of at least 3 g/day) and a topical one (rectal enema or possibly suppository) should be used [3, 28–37]. The usefulness of local treatment in the extensive form (E3) is sometimes put into question, but is aimed at reducing inflammation in the rectum, which is the most important factor responsible for the symptoms with a significant impact on quality of life, such as faecal urgency or incontinence [3, 9, 36, 37].
Mesalazine can be recommended both in a single daily dose (especially for prolonged-release preparations) as well as in divided doses – the efficacy in both cases is similar, while treatment compliance increases in a single-dose regimen [6, 38, 39]. There are clinical data justifying the use of higher oral doses of mesalazine than 4 g daily, which is usually considered the maximum level, in highly selected clinical situations [40–42].
Mesalazine is safe in long-term therapy. However, because of the risk of nephrotoxicity, renal function (blood creatinine and urinalysis) should be monitored before and during therapy [3, 12].
An alternative to mesalazine is sulfasalazine [3, 13].
If no remission has been achieved after the use of mesalazine, the therapeutic indications should first be verified (disease activity may justify the use of agents with a greater anti-inflammatory potential), then it should be made sure that the treatment is carried out optimally (dose and route of administration), and if not, it should be optimised by increasing the dose of the drug or using combination therapy, and finally the differential diagnosis should be extended by other possible causes of symptom exacerbation than UC (underlying infections, cancer) [3, 9, 11, 12].
5. We recommend maintenance treatment with mesalazine in patients in whom remission was achieved with mesalazine.
(Quality of evidence: low; strength of recommendation: strong)
| Recommendation #5 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 8% | 92% | ||||
If in mild-to-moderate exacerbation of UC remission was obtained with mesalazine, the same agent should also be used as maintenance therapy. In the case of UC confined to the rectum (proctitis) it is usually recommended to apply mesalazine topically (suppositories); in the left-sided form it should be used as oral and topical formulations (suppositories, less frequently rectal enemas), and in the extensive form oral formulations of mesalazine are recommended. The lowest recommended oral dose is 2 g/day [3, 9, 11, 12, 43–49].
6. If remission is not achieved with mesalazine, we recommend using topical (budesonide) or systemic (prednisone, methylprednisolone) steroids. The choice of the specific type of steroids should depend mainly on the severity of symptoms.
(Quality of evidence: low; strength of recommendation: strong)
| Recommendation #6 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 34% | 66% | ||||
If remission has not been obtained despite the optimal use of mesalazine, treatment with topical (budesonide) or systemic oral (prednisone, methylprednisolone) steroids should be considered [3, 9, 11, 50–54].
Budesonide is a steroid with potent topical anti-inflammatory activity, characterised by a high first-pass effect (over 90% of the drug is metabolised during the first pass in the liver), owing to which its systemic adverse effects are very limited. Budesonide in the MMX form that releases the active substance in the large intestine is used in the treatment of UC. The medicine is available in the oral form dosed at 9 mg/day. The indication for the use of budesonide is induction treatment of mild to moderate disease, and its efficacy has been best documented in the case of left-sided location of inflammatory lesions. The duration of treatment is usually 8 weeks. Budesonide does not require tapering before discontinuation. Budesonide should not be used as maintenance therapy [3, 9, 11, 50–52].
Prednisone (0.5–1 mg/kg, usually 40 mg/day) or methylprednisolone is usually used in moderate-to-severe UC. They are characterised by a very high anti-inflammatory potential and rapid onset of action but have systemic adverse effects typical for steroids [3, 9, 11–13, 53, 54]. They are used in induction treatment for 2–4 weeks, and then must be tapered off slowly. The entire course of treatment should last no more than 8–12 weeks. Steroids should be used at the target dose from the start – initiating treatment with low doses with subsequent up-titration in the absence of improvement is not recommended [3, 9, 11].
Steroids should be used for induction therapy; they should not be used as maintenance therapy [3, 9, 11].
The choice between topical and systemic steroids depends on the severity of the clinical symptoms. In cases with lower activity, and/or with partial improvement after the use of mesalazine, budesonide is the preferred drug. On the other hand, when symptoms are more severe and no improvement is obtained after the use of mesalazine, prednisone or methylprednisolone should be used [3, 9, 11–13].
7. We recommend maintenance treatment with mesalazine in patients in whom remission was achieved with steroids and mesalazine.
(Quality of evidence: low; strength of recommendation: strong)
| Recommendation #7 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 16% | 84% | ||||
If in UC with mild-to-moderate activity remission was obtained with steroids and mesalazine, mesalazine alone can be used as maintenance therapy. This applies to the first and subsequent exacerbations of UC, provided that exacerbations are rare and no risk factors for a severe course of the disease are present [3, 9, 11, 43–45].
If prolonged steroid treatment was required to achieve remission or disease activity was high at baseline, or when exacerbations occur frequently, or when previous maintenance therapy with mesalazine did not provide adequate disease control, the addition of thiopurines to mesalazine in maintenance therapy should be considered [3, 9, 11–13].
8. We recommend treatment with immunosuppressants, biological agents or tofacitinib in patients with steroid-dependent or steroid-refractory ulcerative colitis.
(Quality of evidence: moderate; strength of recommendation: strong)
| Recommendation #8 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 25% | 75% | ||||
In the case of steroid-refractory UC – inefficacy of steroids in induction therapy – medicines of the subsequent line of therapy should be used. In patients with mild-to-moderate disease activity and partial but incomplete improvement after steroid use, treatment with thiopurines may exceptionally be considered. However, these medicines need a long time to achieve an optimal therapeutic effect, so they should not be used when the activity of the disease requires the treatment to be rapidly effective. Therefore, if the patient’s clinical status does not allow one to wait for the therapeutic effect of thiopurines, the use of biological agents or tofacitinib should be considered [3, 9, 55–58].
In the case of steroid-dependent UC – when remission is achieved with steroids (topical or systemic) but exacerbation occurs during dose reduction or within 3 months after the end of steroid therapy – thiopurine is preferred in patients with UC with mild to moderate activity, but in selected cases the use of biologic agents or tofacitinib may also be considered as an alternative [3, 9, 13, 55–58].
Azathioprine (2–2.5 mg/kg) or mercaptopurine (1–1.5 mg/kg) is administered orally, in one or two divided doses. The time to the full therapeutic effect of thiopurines is rather long (6–12 weeks); therefore, in order to achieve earlier control of symptoms, steroid treatment at the lowest effective dose should be maintained (for another 4–8 weeks) and then an attempt should be made to taper them off (but treatment with systemic steroids should not last longer than 12 weeks). The recurrence of symptoms despite sufficiently long use of thiopurines at optimal doses suggests the lack of their efficacy and is an indication for the use of tofacitinib or biological agents [3, 9, 11–13].
Because of the risk of adverse effects during thiopurine therapy, laboratory parameters – mainly blood cell count, alanine aminotransferase (AlAT) and creatinine – should be monitored periodically, optimally every 2 weeks during the first 2 months of treatment and then at least every 3 months. In view of an increased risk of non-melanoma skin cancer and cervical cancer, all patients treated with thiopurines should be under the constant care of a dermatologist and women should participate in a cervical cancer prevention programme. In addition, because of a slight increase in the risk of aggressive B-cell lymphoma, some experts do not recommend the use of thiopurines in patients not previously infected with Epstein-Barr virus (EBV). Therefore, prior to initiating thiopurine therapy, we suggest determining whether the patient has had infectious mononucleosis or to check the EBV serological status. This recommendation applies especially to young men (< 35 years of age) [3, 11–14].
To monitor thiopurine treatment and to diagnose the causes of their weak therapeutic effect or adverse effects, it may be helpful to measure the levels of 6-thioguanine (an active metabolite) and 6-methylmercaptopurine (a metabolite responsible for some side effects) in erythrocytes. Finding a reduced level of 6-thioguanine in a patient with a weak therapeutic effect may indicate that the patient does not use the drug or imply the need to optimise the thiopurine dose. On the other hand, the normal (therapeutic) level will indicate the need to switch the medicine to another one with a greater therapeutic potential. Alternatively, the thiopurine methyltransferase level in erythrocytes may also be determined prior to the initiation of thiopurines. The absence or low activity of this enzyme is a contraindication to thiopurine treatment [3, 14].
9. Thiopurines should not be used for induction therapy.
(Quality of evidence: low; strength of recommendation: strong)
| Recommendation #9 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 8% | 92% | ||||
Due to the mechanism of action of thiopurines (antimetabolites of purine bases), the target therapeutic effect can be achieved after 6–12 weeks of treatment; therefore they should not be used for induction therapy [3, 9, 11–13, 56–58].
10. Among targeted therapies, anti-TNF antibodies, vedolizumab, ustekinumab or tofacitinib may be used as the drugs of first choice (if conventional therapy proved ineffective or is not tolerated), and also in the case of primary nonresponse or loss of efficacy of another targeted treatment. The choice of a specific therapeutic agent depends on the patient’s profile.
(Quality of evidence: moderate; strength of recommendation: weak)
| Recommendation #10 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 34% | 66% | ||||
Targeted therapies include biological agents (anti-TNF, vedolizumab, ustekinumab) and also new small molecule drugs [3, 9].
In mild-to-moderate UC, targeted therapies should be considered in the following cases: failure of treatment with the medicines used for induction treatment (in particular in patients with steroid-refractory disease), consecutive relapses despite optimal maintenance treatment (in particular in patients with steroid-dependent disease), or intolerance or adverse effects of the previously used conventional therapy (mesalazine, steroids, immunosuppressants) [3, 9, 11–13].
Targeted therapies can be used regardless of the extent of inflammatory lesions. The decision to use them will be made earlier in patients with extensive UC, but the lack of effectiveness of standard treatment in the case of proctitis is also an indication for the use of a medicine from this group [3, 9, 11–13].
On the basis of the currently available results of clinical trials, any of the targeted agents (when indicated) can be used as the drug of first choice for this form of the disease. The choice should take into account the patient’s profile (risk of adverse effects, comorbidities, disease history, presence of extraintestinal symptoms). An important aspect is the route of administration of the medicine and related patient’s preferences. At present, we have molecularly targeted agents administered orally, subcutaneously and intravenously [3, 9, 11].
In the case of the primary non-response to any agent, a switch to an agent with a different mechanism of action should be considered. Loss of response is primarily an indication for intensification of treatment, and, in the absence of improvement, for changing the class of drug [3, 9, 11].
Anti-TNF agents (anti-TNF-α monoclonal antibodies) used in UC include infliximab, adalimumab and golimumab. In Poland infliximab is by far most commonly used in practice, for administrative reasons [3, 9, 11].
Infliximab is a human/mouse chimeric antibody of the IgG1 class. It is administered intravenously at a dose of 5 mg/kg in a 0–2–6 week regimen for induction therapy and every 8 weeks thereafter for maintenance therapy. Recently, a subcutaneous formulation of infliximab has also been approved in Europe (as of December 2022). This medicine is characterised by a very fast and potent onset of action along with a good safety profile, but also high immunogenicity. The most common side effects include hypersensitivity reactions and an increased infection risk, and also a slight increase in the cancer risk has been suggested (this mainly applies to the risk of skin melanoma). In some cases, it may be useful to intensify the treatment (by increasing the dose to 10 mg/kg every 8 weeks or 5 mg/kg every 4 weeks). Concomitant use of thiopurines reduces the risk of developing antibodies against infliximab, which improves the efficacy of therapy. This effect is strongest in the first year of combination treatment [3, 9, 11–13, 59, 60].
Adalimumab is a human antibody of the IgG1 class, administered subcutaneously in a regimen of 160–80–40 mg every 2 weeks. Induction treatment lasts 12 weeks. It is less immunogenic than infliximab (adalimumab may be considered if infliximab is ineffective), and apart from that it has similar properties [3, 9, 11–13, 61–63].
To optimise the effectiveness of infliximab and adalimumab therapy, some experts recommend therapeutic drug monitoring including the assessment of trough serum drug levels with potential testing for neutralising anti-drug antibodies. Such an approach may be useful especially in the case of loss of response and enables personalised modifications of drug dosing, based on the test results [3].
Vedolizumab is a humanised anti-α4β7 integrin antibody. Vedolizumab is administered first in induction therapy by intravenous infusion (300 mg in a 0–2–6 week regimen), and then in maintenance therapy by intravenous infusion (300 mg every 8 weeks). An additional dose of the drug is allowable at week 10 after treatment initiation if no discernible clinical benefit has been obtained after 3 induction doses. Also maintenance therapy dosing can be intensified, by administering 300 mg intravenously every 4 weeks, depending on the clinical presentation. A subcutaneous dosage form of vedolizumab is available for maintenance treatment. The drug at a dose of 108 mg administered every 2 weeks can be used in patients who have achieved remission with the intravenous form (at least 2 intravenous infusions, but a possible change in the route of administration is recommended in patients in stable remission). In comparison with infliximab, vedolizumab is characterised by lower immunogenicity, lower infection risk, and higher oncological safety [3, 9, 11–13, 64–66].
Ustekinumab is an antibody against the p40 subunit common to IL-12 and IL-23. The medicine is administered in a single intravenous weight-dependent dose, and then in subcutaneous doses of 90 mg every 8 or 12 weeks. Ustekinumab is characterised by a good safety profile and low immunogenicity [3, 9, 11–13, 67].
Tofacitinib is a small molecule drug that non-selectively inhibits Janus kinases. It is administered orally at an initial dose of 2 × 10 mg/day for 8 weeks, followed by a maintenance dose of 2 × 5 mg. In selected cases, the 2 × 10 mg induction therapy may be extended for up to 16 weeks. In patients with a reduced response to the 2 × 5 mg maintenance dose and with a low risk of venous thromboembolic complications, the dosage can be increased to 2 × 10 mg/day, using this regimen for the shortest possible time. Tofacitinib has a rapid onset of action and a good safety profile (similar to infliximab) and is therefore used for induction and maintenance therapy. Possible side effects include infections (especially shingles/herpes zoster); special caution is also required in patients with a high risk of thromboembolic complications. Because it is a small molecule medicine, it does not induce the production of neutralising anti-drug antibodies [3, 9, 11–13, 68, 69].
Moderate-to-severe activity
11. We recommend systemic steroids and mesalazine as the treatment of first choice for induction of remission.
(Quality of evidence: low; strength of recommendation: strong)
| Recommendation #11 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
In the case of a moderate-to-severe activity, mesalazine at high doses is recommended for induction therapy (in the oral form and – if tolerated – in the topical form), in combination with systemic steroids (at the standard dose). Steroid therapy should be started from the target dose. Induction therapy with steroids is conducted for 2–4 weeks, after which the drug from this class should be slowly tapered off so that the entire course of treatment lasts no more than 12 weeks. In some cases of moderate activity UC without additional risk factors the use of budesonide is allowed [3, 9, 11–13, 54].
12. We recommend maintenance treatment with thiopurines in patients in whom remission was achieved with steroids. In each patient mesalazine should be additionally used as part of maintenance therapy, if there are no contraindications.
(Quality of evidence: low; strength of recommendation: strong)
| Recommendation #12 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
After clinical remission is obtained with steroids in a patient with moderate-to-severe UC, thiopurines and mesalazine should be used for maintenance therapy. The slow onset of action of thiopurines should be taken into account and treatment with these drugs should be started as early as possible [3, 9, 11–13, 56]. However, in the case of exacerbation in patients already treated with thiopurines or who have contraindications or are intolerant to this drug class and require repeated courses of steroid therapy (even if the criteria of steroid dependency are not met), as well as in patients with risk factors for the adverse course of UC, the use of targeted therapies should not be delayed [3, 9, 11–13, 70]. According to AGA, the most important risk factors for complicated UC course include age < 40 years at the time of diagnosis, high endoscopic activity (usually understood as the presence of deep ulcers), the need for hospitalisation for UC exacerbation, extensiveness of lesions, and elevated inflammatory markers (CRP) [70].
One of the objectives of long-term use of mesalazine for maintenance therapy is the chemoprevention of colorectal cancer [3].
13. We recommend treatment with an anti-TNF agent, vedolizumab, ustekinumab or tofacitinib in steroid-refractory, steroid-dependent and/or steroid-intolerant patients.
(Quality of evidence: high; strength of recommendation: strong)
| Recommendation #13 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
In the event of steroid-refractory, steroid-dependent disease or steroid intolerance in patients with moderate-to-severe UC, targeted therapies (biologics or small molecule drugs) should be used [3, 9, 11–13]. Only in selected cases of steroid dependency, in patients with a previously mild course of the disease, may the use of thiopurines be exceptionally considered. As already mentioned, currently it is not possible to clearly determine which of the targeted agents should be used as the drug of first choice. Nevertheless, it should be borne in mind that the use of each subsequent drug medicine is associated with a lower chance of achieving remission. Drug selection should take into account the profile and preferences of the patient [3, 9, 11–13].
On the basis of available data, the duration of targeted therapy cannot be clearly established. It should be assumed that in the case of moderate-to-severe activity, well-tolerated treatment should be carried out in a long-term manner [3, 9, 11–13].
Acute severe ulcerative colitis (ASUC)
Acute severe ulcerative colitis (ASUC) is characterised by high-activity inflammatory lesions in the large intestine with severe intestinal symptoms and an accompanying systemic response. Even now it is still associated with a high risk of colectomy and a relatively high mortality rate. For this reason, the condition should be diagnosed as soon as possible and appropriate treatment should be initiated without delay. Treatment of ASUC should take place in a hospital setting [3, 10–13, 71–74].
Truelove and Witts criteria are used to diagnose ASUC. On their basis, relying on a medical interview, physical examination and basic laboratory tests, it is possible to quickly identify a patient who requires intensive treatment in a hospital setting. The basis for the diagnosis is the presence of ≥ 6 bloody stools per day accompanied by at least one of the following systemic reaction indicators: haemoglobin (Hb) < 10.5 g/l, erythrocyte sedimentation rate (ESR) > 30 mm/h (or CRP > 30 mg/l), body temperature above 37.8°C or tachycardia > 90 bpm [71–73].
To confirm ASUC, it is recommended to perform an endoscopic examination – rectosigmoidoscopy without preparation. The initial assessment of the patient with ASUC should also include plain X-ray of the abdomen (to exclude toxic megacolon) and microbiological tests to rule out an underlying infection causing exacerbation (primarily tests for infection with the toxigenic C. difficile) as well as laboratory tests (primarily peripheral blood cell count, electrolyte levels, CRP, creatinine). Other examinations (e.g. abdominal ultrasound, computed tomography) depend on the clinical situation [1, 3, 10–13].
A particularly challenging clinical situation is ASUC as the first manifestation of UC. In such a case it should be borne in mind that the diagnosis of UC is based on the overall clinical presentation, and thus the absence of the histopathological confirmation when other clinical criteria are met cannot be the reason for delaying the start of adequate treatment [1, 3, 10–13].
14. We suggest intravenous steroid treatment in the hospital setting of patients who meet the Truelove and Witts criteria for acute severe ulcerative colitis.
(Quality of evidence: very low; strength of recommendation: weak)
| Recommendation #14 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
Each patient with symptoms of severe exacerbation of UC should be evaluated for ASUC according to the Truelove and Witts criteria. Further treatment of patients with confirmed ASUC is carried out in a hospital setting. The drugs of first choice are systemic steroids administered intravenously (hydrocortisone 300–400 mg/day in divided doses or methylprednisolone 40–60 mg/day) [3, 10–13]. Plain X-ray of the abdomen should always be performed to rule out toxic megacolon along with microbiological testing to rule out an underlying infection. Waiting for the results of microbiological tests should not delay the start of steroid therapy. In view of the increased risk of thromboembolic complications, each patient should be administered low-molecular-weight heparin at a prophylactic dose. Antibiotic therapy is sometimes also used adjunctively in selected patients [1, 3, 10–13].
15. We recommend infliximab in patients who have not responded to 3 days of intravenous steroid therapy. As an alternative to infliximab, ciclosporin may be used.
(Quality of evidence: moderate; strength of recommendation: strong)
| Recommendation #15 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 34% | 66% | ||||
The efficacy of intravenous steroid therapy for ASUC should be evaluated after 3 days of its use. The lack of improvement is recognised when the patient has more than 8 bowel movements per day or 3–8 bowel movements accompanied by a high CRP level of above 45 mg/l (the Oxford criteria) [1, 3, 73].
If the criteria for no improvement are met, infliximab (at a standard dose of 5 mg/kg) should be considered. If clinical improvement is achieved after a further 3–5 days, infliximab treatment should be continued (subsequent doses at 2 and 6 weeks after the first dose, followed by maintenance therapy every 8 weeks), the intravenous steroid should be replaced with its oral formulation at the target dose (prednisone 0.5–1 mg/kg, usually 40 mg/day), and after a further 2–4 weeks steroid tapering should be initiated. In selected cases (especially in patients with a high risk of complications of multidrug immunosuppressive therapy), definitive discontinuation of ineffective intravenous steroid therapy without switching to the oral form may be considered, but provided that an evident improvement was obtained with infliximab. Moreover, mesalazine treatment should be continued, azathioprine treatment should be continued in patients previously treated with this agent and initiation of azathioprine treatment in azathioprine-naïve patients should be considered (due to greater efficacy of the combination treatment with infliximab and thiopurine) [1, 3, 10, 73–79].
There are single studies to support an additional dose of infliximab approximately 7 days after the first dose in patients who have had only a partial clinical response. Nevertheless, no clear recommendations can currently be provided for the indications, timing and dosage of such an infusion. Similarly, some authors suggest a higher dose of infliximab of 10 mg/kg, as a rescue therapy. However, also in this case we do not have sufficient evidence to routinely recommend this dosage [3, 10–13, 80–83].
As an alternative to infliximab, ciclosporin can be used in some patients (2 mg/kg/day intravenously). However, the higher number of contraindications in comparison with infliximab, lower tolerability and the need for further maintenance therapy with thiopurines (ciclosporin is not used as maintenance therapy and should be discontinued after 3–6 months of treatment) should be taken into account with ciclosporin. Moreover, ciclosporin should not be used in patients who developed ASUC during maintenance therapy with thiopurines. Ciclosporin treatment should be guided by its serum levels (the target level is 100–200 ng/ml for maintenance therapy). After 3–5 days of intravenous ciclosporin treatment, in patients with a clinical improvement the intravenous route should be replaced by the oral one (4–5 mg/kg in two divided doses), the intravenous steroid should be replaced with its oral formulation at the target dose (prednisone 0.5–1 mg/kg, usually 40 mg/day), and after a further 2–4 weeks steroid tapering should be initiated. In selected cases (especially in patients with a high risk of complications of multidrug immunosuppressive therapy), definitive discontinuation of ineffective intravenous steroid therapy without switching to the oral form may be considered, but provided that an evident improvement was obtained with ciclosporin. Moreover, treatment with mesalazine should be continued and azathioprine should be initiated. In total, the combined oral treatment with azathioprine and ciclosporin is usually carried out for about 8–12 weeks, until the full therapeutic effect of the thiopurine is achieved, after which ciclosporin should be discontinued. During treatment with steroids at tapered doses, azathioprine and oral cyclosporine, chemoprophylaxis of Pneumocystis jiroveci infection by trimethoprim-sulfamethoxazole administration should be considered [1, 3, 10–13, 78, 84–92].
The available results of clinical trials demonstrated similar efficacy of ciclosporin and infliximab in the treatment of ASUC in short-term follow-up. However, because of the higher number of contraindications to ciclosporin, its worse tolerability and the need to monitor drug concentrations, a significant decrease in the cost of infliximab therapy after the introduction of biosimilars, and above all the need to discontinue ciclosporin after induction therapy, infliximab is the preferred treatment for ASUC [1, 3, 10, 89–92].
Available data do not support the use of ciclosporin in the absence of a response to infliximab or vice versa, but in exceptional cases this is allowable if the patient’s clinical condition makes it possible to postpone surgery, if required. In this case, however, if colectomy is necessary, the risk of intra- and postoperative complications may be particularly increased [3, 10–13].
It should be emphasised that a conservatively treated patient with ASUC should be monitored on an ongoing basis for emergency indications for surgical treatment [3, 10–13].
16. We suggest surgery if no response is achieved after 5 consecutive days of treatment with ciclosporin or infliximab. Surgical treatment should always be considered in a patient with symptoms of toxic megacolon, massive bleeding and/or signs of shock.
(Quality of evidence: low; strength of recommendation: weak)
| Recommendation #16 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
The lack of clinical response after 5 days of rescue treatment with infliximab or cyclosporine is an indication for surgical treatment – colectomy. An indication for earlier surgery is toxic megacolon, massive bleeding, shock or perforation [3, 10–13, 93–98].
If clinical improvement is obtained but without full remission (provided that the systemic inflammatory reaction has been reduced), it is allowable to continue rescue treatment for another 5–7 days or, in exceptional cases, to switch to ciclosporin if infliximab was previously used or vice versa. However, the evidence justifying the switch is contradictory and of poor quality, and with the failure of second-line rescue therapy, the risk of surgical treatment complications increases significantly [3, 10–13].
In addition, for patients with previous failure of anti-TNF-α therapy, we have data from a small number of observational (mostly retrospective) studies that suggest the possibility of using vedolizumab or ustekinumab (instead of thiopurines) as maintenance therapy in patients successfully treated with ciclosporin for ASUC [99, 100].
There is also an increasing number of reports describing cohorts of patients after a previous failure of biologic therapy who were effectively treated with tofacitinib for ASUC [101, 102]. It should be noted, however, that at the time this document was prepared, none of the scientific societies recommended the use of Janus kinase inhibitors in this clinical scenario.
17. We recommend maintenance treatment with infliximab in patients in whom a response to infliximab has been obtained.
(Quality of evidence: moderate; strength of recommendation: strong)
| Recommendation #17 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
In patients in whom a clinical response was obtained in ASUC with the use of infliximab, this drug should be used according to the standard dosage regimen, including in maintenance therapy [3, 10–13]. In patients who have been treated so far with thiopurines, this therapy should be continued. The combination of infliximab and thiopurine has been demonstrated to be associated with a lower risk of developing antibodies to the biological agent and to be more effective, even if the occurrence of ASUC during maintenance therapy may indicate the inefficacy of thiopurines. The beneficial effect of this combination treatment decreases over time, and concerns about the safety of such dual therapy when used in long term are increasing. Therefore, in patients with sustained remission discontinuation of thiopurines should be considered after 1–2 years of combination treatment [3, 10–13].
The initiation of thiopurine therapy in previously untreated patients raises more doubts, but the arguments cited above justify such a procedure.
18. We suggest maintenance treatment with thiopurines in patients in whom a response to ciclosporin has been obtained.
(Quality of evidence: very low; strength of recommendation: weak)
| Recommendation #18 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 8% | 92% | ||||
Ciclosporin is used for induction therapy of ASUC but it is not suitable for long-term administration. Thiopurines are used for maintenance therapy in patients in whom a response to ciclosporin has been obtained. In patients who developed ASUC during maintenance therapy with optimally dosed thiopurines, a very high risk exists of another exacerbation after ciclosporin discontinuation. Therefore, ciclosporin rescue therapy is not recommended in such cases [3, 10–13].
As mentioned in the comment on Recommendation No. 16, there is only isolated source of evidence, so far of low quality, that vedolizumab or ustekinumab (instead of thiopurines) can be used as maintenance therapy in patients who have been successfully treated with ciclosporin, but these observations mainly apply to patients with previous failure of anti-TNF-α therapy [99, 100].
Novel medicines
19. Approval of new therapeutic agents can significantly improve the possibilities of treatment in UC. Ozanimod (sphingosine-1-phosphate receptor modulator) is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response, lost response, or were intolerant to either conventional therapy or a biologic agent. The place of novel selective Janus kinase inhibitors such as upadacitinib and filgotinib in the UC treatment algorithm appears to be similar to that of tofacitinib.
(Quality of evidence: moderate; strength of recommendation: strong)
| Recommendation #19 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
The consequence of numerous clinical trials is the approval of new drugs for the treatment of UC. Recently approved medicines include new selective inhibitors of Janus kinases (selective inhibition of JAK1 kinase, a crucial enzyme in the pathomechanism of UC, is designed to provide a better safety profile, with similar efficacy, in comparison with a non-selective inhibitor) and ozanimod [103–109].
Ozanimod belongs to small-molecule drugs with a novel mechanism of action. It is a sphingosine-1-phosphate receptor modulator. Sphingosine-1-phosphate is a phospholipid, a component of cell membranes, which is a ligand for 5 different receptors. It plays an important role in the process of lymphocyte migration and is considered an important element of the pathomechanism of many autoimmune diseases. By modulating the action of two isoforms of the phosphate sphingosine-1 receptor, ozanimod reduces the migration of lymphocytes from lymph nodes into the systemic circulation. In this way it reduces the number of inflammatory infiltration cells in peripheral tissues, including the large intestine.
Ozanimod is successfully used in the treatment of multiple sclerosis and it was approved for the treatment of moderately to severely active UC in patients with inefficacy or intolerance of conventional therapy.
Ozanimod was demonstrated to be effective in UC treatment, for example in the True North study, where its efficacy was proven in both induction and maintenance therapy. A good safety profile and treatment tolerability have been demonstrated. The most common adverse effects of the treatment were infections and elevated levels of liver enzymes.
Ozanimod is taken orally, once daily, at an initial dose of 0.23 mg for the first 4 days, followed by 0.46 mg for the next 3 days, and then at a dose of 0.92 mg starting on the eighth day in long-term treatment.
Ozanimod is contraindicated, for example, in patients with significant cardiac disorders (including patients with cardiac arrhythmia, severe heart failure, recent acute coronary syndrome), liver failure and cancer, and also in pregnancy [103–105].
Upadacitinib is a selective inhibitor of one of the Janus kinases, JAK1. The efficacy of upadacitinib in the treatment of UC has been demonstrated, for example, in the U-ACHIEVE and U-ACCOMPLISH studies. The medicine was effective in both remission induction and maintenance therapy. Upadacitinib is characterised by a good safety profile (probably similar to that of tofacitinib).
It is used orally, at a single dose of 45 mg for 8 weeks in induction therapy (treatment can be extended for another 8 weeks if only partial treatment response is obtained), then at a dose of 15 mg or 30 mg in maintenance therapy.
Upadacitinib is contraindicated e.g. in patients with active infections, hepatic impairment and in pregnancy. The observed adverse reactions include infections, including opportunistic ones (especially shingles/herpes zoster), lymphopenia, neutropenia and elevated liver transaminases [106–108].
Filgotinib is another selective inhibitor of JAK1. The efficacy of filgotinib in the treatment of active UC was demonstrated, for example, in the SELECTION study. The medicine is used at a single daily dose of 200 mg for induction therapy conducted for 10 weeks (remission induction may be prolonged by 12 weeks in justified cases) and for maintenance therapy. The safety profile and adverse reactions are similar to those of upadacitinib [109].
The position of the novel Janus kinase inhibitors in the treatment algorithms for patients with UC seems to be similar as that of tofacitinib [108].
Surgical treatment
20. Elective, urgent or emergency indications for surgical treatment of ulcerative colitis are possible. The most commonly performed procedure is restorative proctocolectomy with ileal pouch-anal anastomosis, which in centres with adequate experience in minimally invasive techniques in abdominal surgery can also be carried out laparoscopically.
| Recommendation #20 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 7% | 7% | 86% | |||
Surgical treatment of UC can be taken into consideration at any stage of the disease. Whenever possible, minimally invasive, laparoscopic techniques are preferred in centres with adequate experience with these techniques [3, 6, 10, 12, 13].
In a patient with massive haemorrhage with haemodynamic consequences, intestinal perforation or toxic megacolon with septic symptoms, surgical treatment should be undertaken immediately, in an emergency mode. The most common indication for urgent surgery (within a few days) is acute severe UC (ASUC) which does not respond to the rescue therapy (usually involving administration of parenteral steroids and infliximab or ciclosporin). Elective indications include the lack of full efficacy and/or adverse effects of pharmacological treatment used so far in a patient with severe or moderately severe UC (but not meeting the ASUC criteria), the presence of precancerous lesions or colorectal cancer or the presence of chronic colorectal strictures (especially symptomatic) of an unclear and difficult-to-determine nature (inflammatory stricture? cancer?) [3, 10, 12].
The most commonly performed type of surgery is restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA), i.e. with the restoration of gastrointestinal tract continuity. This procedure should be performed in centres with adequate experience with this type of surgery and is usually performed in several steps. Before a decision is made to carry out IPAA, the patient should be informed about the risk of early and late complications. According to various analyses, the incidence of early complications, appearing up to 30 days from the date of surgery, is 9–65%, while the incidence of late complications is 3–55%. The most common complications include ileal pouch inflammation, postoperative wound infection, and ileus or subileus [3, 10].
In emergency cases, Hartmann colectomy is the preferred surgery procedure, especially in debilitated patients exposed to steroids for a long time [3, 10].
In exceptional situations, it is allowable to perform colectomy with preservation of the rectum and ileorectal anastomosis. This especially applies to patients with minimal severity of inflammatory lesions in the rectum or, for example, to women intending to get pregnant in the future. We have data suggesting that this type of procedure is better than IPAA in terms of functional results understood as the number of bowel movements per day or the number of nocturnal bowel movements. It should be emphasised, however, that preservation of the rectum is associated with an increased risk of oncological complications (precancerous lesions, rectal cancer); hence strict endoscopic surveillance following this type of surgery is necessary (the principles of such surveillance have not been clearly defined) [3, 6, 10].
A procedure with a lower risk of intra- and postoperative complications than IPAA is proctocolectomy with end-ileostomy. This approach may represent an alternative to IPAA, and before a decision is made on the extent of surgical intervention, the patient should always be informed about the available treatment options, including their advantages and disadvantages [3, 6, 10].
21. The risk of intra- and postoperative complications is increased in patients under chronic steroid therapy and with malnutrition. Mesalazine and thiopurines have no impact on this risk, while data on TNF-α antagonists are inconclusive. We do not have any evidence for negative effects of other monoclonal antibodies (vedolizumab, ustekinumab), and in the case of small molecule drugs (Janus kinase inhibitors, ozanimod) this risk has not yet been clearly determined.
| Recommendation #21 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 25% | 75% | ||||
Regardless of the type of surgery, proper preparation of the patient is very important for treatment outcomes. In a broader sense, it is necessary to implement the principles of prehabilitation of the patient, understood as an improvement of the patient’s metabolic, physical and mental status [3, 6, 10, 14, 110].
The main elements to be taken into account in a patient planned for surgical intervention are: prevention and/or treatment of malnutrition, optimisation of the existing immunosuppressive therapy, treatment of anaemia, and prevention of thromboembolic complications [10, 110].
Malnutrition, very common in patients with UC, increases the risk of intra- and postoperative complications up to several times. In each case of a patient with UC being prepared for surgical treatment, the assessment of nutritional status is mandatory. There is evidence, mainly from observational studies, that preoperative enteral and/or parenteral nutrition in patients with nutritional deficits improves the safety of surgical treatment [3, 6, 10, 11, 14, 110].
The principles of nutritional intervention should be adapted to the clinical situation. In adults it may be preferable to supplement a balanced oral diet with ready-made, pharmaceutical supplemental formulas (supplemental enteral nutrition). If clinically necessary, partial and total enteral nutrition is also allowable, and if there is no other option, parenteral nutrition can be used [3, 14, 111]. According to the recommendations of European Society for Clinical Nutrition and Metabolism (ESPEN), such a nutritional intervention should be carried out for about 7–14 days or longer in the case of particularly severe malnutrition. Also anaemia (Hb < 13 g/dl in men and Hb < 12 g/dl in women) worsens the surgical outcomes. Treatment of iron deficiency anaemia in the context of surgical treatment should primarily consist in intravenous iron supply [3, 14, 111].
The drug class that is most significantly determinant for the results of surgical treatment is steroids, which increase the risk of infectious complications and anastomosis leaks. We have clear evidence that with steroids this risk is at least doubled [3, 6, 10, 14, 112]. The safe dose of steroids is not well defined. Therefore, whenever possible, surgical treatment in UC should be postponed until maximum dose reduction or complete discontinuation of the steroid is achieved – this is especially true for IPAA [6, 10]. There is no convincing and conclusive evidence that patients receiving these medicines and undergoing surgery require an additional dose (stress dose) of steroids perioperatively. However, in the case of chronic steroid use (for > 4 weeks) and the impossibility of steroid discontinuation before surgery, treatment should be continued postoperatively (usually intravenously in the perioperative period if the patient remains fasting, and then orally) with a steady dose reduction – faster when shorter steroid therapy was conducted before surgery [3, 6, 10, 14].
There is no evidence that thiopurines or calcineurin inhibitors increase the risk of intra- and postoperative complications [10]. Most scientific evidence also indicates that anti-TNF-α agents can be safely used in patients undergoing surgery, although the first systematic reviews and meta-analyses in this area were inconclusive [3, 10, 14, 113]. The main factor determining the timing of surgical treatment in a patient with UC receiving anti-TNF therapy is the patient’s clinical condition and indications for surgical treatment. The ECCO guidelines only suggest that IPAA procedures in patients exposed to biological drugs (the available data are mainly for TNF-α antagonists) should be performed in several steps due to the potential increase in the risk of complications associated with ileal pouch surgery [10].
The data on safety of vedolizumab and ustekinumab in this context are very limited, but these medicines do not seem to increase the risk of intra- and postoperative complications [10]. There are no relevant data for small molecule drugs (Janus kinase inhibitors and ozanimod), but their short half-life suggests that they will not have a significant negative impact on the surgical outcome [10].
In each patient with active UC (including when eligible for surgical treatment) it is necessary to assess the indications for the prophylaxis of thromboembolic complications since they are one of the main causes of mortality in patients with UC. What is important to mention, there is no conclusive evidence that such a strategy (usually involving the use of low-molecular-weight heparin at a preventive dose) increases the risk of bleeding during and after surgery [3, 6, 10, 110].
Patients with UC undergoing surgical treatment, especially those receiving previous chronic steroid therapy and malnourished, should be treated as patients with a high risk of surgical site infection and the procedure should be modified accordingly (prolongation of preoperative antibiotic prophylaxis or early initiation of antibiotic therapy, use of antibacterial sutures, prophylactic use of closed negative pressure wound therapy) [110].
III. Other
A. Pouchitis
22. Diagnosis of pouchitis is based on the assessment of clinical symptoms, as well as endoscopic and histopathological criteria.
| Recommendation #22 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 29% | 71% | ||||
Pouchitis develops in 25% of patients within 1 year and in 45% of patients within 5 years after restorative proctocolectomy [114]. The pathogenesis of pouchitis is unclear. Risk factors include primary sclerosing cholangitis (PSC), non-smoking, extensive UC and thrombocytosis before surgery, backwash ileitis, use of non-steroidal anti-inflammatory drugs (NSAIDs), the presence of perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) and the coexistence of autoimmune diseases [115].
A patient with an ileal pouch usually passes 4–8 semi-liquid stools daily (4–7 during the day and 1–2 at night); the daily volume of stool is about 700 ml. Pouchitis signs and symptoms are an increase in the amount and volume of stool, faecal incontinence, pain and discomfort in the pelvis, fever, less often extraintestinal symptoms. The appearance of blood in the stool, rare in pouchitis, suggests inflammation of the residual cuff of anorectal mucosa (called cuffitis) [114–116]. The differential diagnosis should also take into account infectious factors (mainly C. difficile, CMV), Crohn’s disease, eosinophilic and autoimmune IgG4-mediated inflammation, ischaemia or stenosis of the pouch, bacterial overgrowth, pouch emptying/motility disturbances, or the use of NSAIDs. To make a diagnosis, it is necessary to perform endoscopic examination with an assessment of the efferent and afferent loop, the distal pouch, the anastomosis, and the rectal remnant or cuff, along with the collection of biopsy specimens. Endoscopic manifestations of pouchitis include oedema, granulation, fragility of the mucosa, loss of the vascular pattern, presence of ulcers, and bleeding. It is recommended to collect 4–6 biopsy specimens even in the absence of macroscopic signs of inflammation. It may be relevant for differential diagnosis to take specimens also from the pre-pouch ileum. The dominant histopathological feature is non-specific inflammatory infiltration with the presence of polymorphonuclear leukocytes, crypt abscesses and ulcers [114–117].
23. In acute pouchitis, we recommend the use of antibiotics (ciprofloxacin, metronidazole) as the treatment of first choice.
(Quality of evidence: moderate; strength of recommendation: strong)
| Recommendation #23 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 16% | 84% | ||||
Depending on the course and adopted criteria, pouchitis can be classified as acute (lasting for < 4 weeks) or chronic (lasting for ≥ 4 weeks); or as antibiotic-responsive, antibiotic-dependent or antibiotic-refractory. In acute pouchitis, the treatment of first choice is antibiotics – ciprofloxacin 2 × 500 mg (preferred because of its better efficacy and tolerability) or metronidazole 3 × 500 mg for 2–4 weeks. Approximately 39% of patients respond to antibiotic therapy and experience only a single episode of pouchitis [118].
In the case of antibiotic intolerance, other treatment options may be considered (probiotics, and especially a probiotic product containing 8 well-studied strains – originally De Simone Formulation, budesonide, mesalazine) [114, 119].
The efficacy of rifaximin in the treatment of acute pouchitis has not been confirmed, although there are data indicating its possible use in this indication [118, 120].
24. For antibiotic-dependent chronic pouchitis, we recommend the use of prolonged antibiotic therapy and selected probiotics as the treatment of first choice.
(Quality of evidence: low; strength of recommendation: strong)
| Recommendation #24 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 28% | 72% | ||||
After the first episode of pouchitis, around 60% of patients have at least one relapse and 20% develop chronic inflammation [121]. If the disease symptoms recur ≥ 4 times a year despite the use of antibiotic therapy, antibiotic-dependent pouchitis can be diagnosed [122]. The mainstay of patient management is prolonged antibiotic therapy. Treatment is often started with those previously used antibiotics, which were clinically effective [119, 123]. There is evidence for the efficacy of combination therapy (ciprofloxacin plus metronidazole, ciprofloxacin plus rifaximin or ciprofloxacin plus tinidazole) used for 4 weeks [114].
In maintenance therapy after a course of standard antibiotic therapy (24-month follow-up), rifaximin 200 mg/day was shown to be effective; its dose may be increased to 1800 mg/day if only a partial response is obtained [124].
In one study, antibiotic therapy (mainly ciprofloxacin and/or metronidazole) was used for at least 12 months and made it possible to obtain remission in 21% of the treated patients, but 28% experienced adverse reactions to antibiotic therapy and in 78% antibiotic resistance (especially to ciprofloxacin) of the bacteria grown in faecal cultures was found [125].
Treatment of antibiotic-dependent pouchitis is challenging because prolonged antibiotic therapy increases the risk of antibiotic resistance and adverse reactions, and thus it is important that selected probiotics (mainly a product containing 8 well-studied strains) have also been demonstrated to be effective in this indication [126, 127].
25. In the case of antibiotic-refractory chronic pouchitis, we suggest the use of budesonide or vedolizumab, or possibly other targeted agents.
(Quality of evidence: moderate; strength of recommendation: weak)
| Recommendation #25 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 14% | 86% | ||||
Vedolizumab is the only medicine approved for the treatment of moderate-to-severe active chronic pouchitis in patients who have responded inadequately or no longer respond to antibiotic therapy. Treatment should be initiated together with antibiotic therapy. Vedolizumab is administered intravenously at a standard dose of 300 mg at weeks 0, 2 and 6 and every 8 weeks thereafter. If no benefit is observed by week 14, treatment discontinuation should be considered [128].
In a multi-centre retrospective study with a median follow-up of 1.3 years, a clinical response was obtained in 71% of the subjects during vedolizumab treatment, with 19.3% achieving clinical remission [129]. A randomised trial (the EARNEST trial) confirmed vedolizumab efficacy in reducing clinical and endoscopic symptoms with good treatment tolerability [130].
Other targeted agents, notably infliximab, adalimumab and ustekinumab, have also been shown to be effective, and benefits of tofacitinib have been reported. Their use can be considered on a case-by-case basis, taking into account the benefits and risks of therapy [114, 119, 123, 131–136].
In the treatment of antibiotic-refractory pouchitis budesonide has also been proven effective, at an oral dose of 9 mg/day for 8 weeks with subsequent tapered discontinuation (effective remission induction in 75% of patients) or administered by rectal enema (6 weeks of treatment with 2 mg/ml) [137–139].
If conservative treatment fails, repeated pouch surgery (which can often be technically difficult or impossible) or pouch removal with end-ileostomy may be considered. An alternative may be the creation of a decompressive loop ileostomy, which is a relatively minimally invasive procedure. However, it should be borne in mind that this type of surgical procedure, i.e. functional shortening of the small intestine, may lead to malabsorption (as in the short bowel syndrome) and may cause renal dysfunction [121–123].
B. Colorectal cancer in UC
26. The basic examination in the surveillance for colorectal cancer in ulcerative colitis patients is colonoscopy with biopsies.
| Recommendation #26 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
The recommended colonoscopic examination performed as part of the surveillance in a patient with UC should have the following features:
Meeting the criteria of high-quality colonoscopy.
High image resolution, the use of dye chromoendoscopy or virtual chromoendoscopy.
Targeted biopsy collection, from visible lesions (alternatively, random biopsies).
Colonoscopy plays the most important role in the prevention of colorectal cancer (CRC), surveillance and detection of early cancer lesions in patients with UC [140, 141]. The efficacy of colonoscopy in reducing CRC incidence and mortality depends on the quality of the examination. Colonoscopy performed in a patient with UC should meet the recognised quality criteria [142]. One of them is bowel preparation. It should be assessed during each examination using the appropriate scale. A relatively simple, widespread and validated scale is the Boston Bowel Preparation Scale (BBPS). Adequate bowel preparation corresponds to a score of at least 6 out of a maximum of 9 points on the BBPS for the assessment of the entire colon (and none of the three evaluated segments should have a score of 0–1 points) [143]. Polyethylene glycol in a split (high-volume (4 l) or low-volume (2 l)) dose is the preferred agent for bowel cleansing before colonoscopy in patients with IBD [144]. Such preparation is similarly tolerated as in people without IBD [145]. Adequate bowel cleansing should be confirmed in 90% of colonoscopies [146].
There are known colonoscopy quality indicators, which depend inter alia on the person performing the examination, with proven importance in CRC surveillance: caecal intubation rate (CIR; optimally ≥ 90%), the percentage of colonoscopies with detection of at least one adenoma – adenoma detection rate (ADR; optimally ≥ 25%), withdrawal time (optimally ≥ 6 min), and a quality index specific to IBD surveillance examinations – the percentage of colonoscopies with detection of at least one dysplastic lesion (NDR, neoplasia detection rate; minimum ≥ 8%) [146–149]. Careful assessment of the mucosa is important. It is widely agreed that colonoscopy should be performed by a physician with expertise in IBD management in addition to endoscopic technical skills [149].
Due to the diagnosis of UC, the long-term course of the disease, the need for repeated endoscopic examinations, and increased levels of anxiety among patients, the ECCO guidelines suggest performing the examinations in patients with IBD under analgosedation, especially when carried out as part of surveillance [150].
Optimally, surveillance colonoscopy should be performed during UC remission [150].
The quality of surveillance colonoscopy is inextricably linked to the available technical facilities. The approach to the surveillance principles evolved together with the evolution of endoscopic techniques. Historically, in surveillance with the use of standard definition white light endoscopy (SD-WLE), the rule was to take random biopsies of the mucosa in four quadrants every 10 cm (which gave a minimum of 32 biopsies) along with targeted biopsies from visible lesions. It has been estimated that if the large intestine of an adult has an average length of 100–150 cm and a circumference of 6–10 cm, then by taking biopsies in this way it was possible to assess histopathologically about 0.001% of its mucosa [151]. However, due to suboptimal image quality, most dysplastic lesions were diagnosed only after examination of randomly collected specimens [152].
The introduction of dye chromoendoscopy (DCE) using such dyes as indigo carmine or methylene blue sprayed onto the intestinal mucosa enabled targeted biopsies from previously invisible lesions. Randomised trials and meta-analyses have demonstrated the superiority of DCE over SD-WLE in the detection of dysplasia [152, 153].
The next step was popularisation of high definition white light endoscopy (HD-WLE). Owing to higher pixel density and image sharpness along with the lower number of artefacts, a significant advantage of HD-WLE in the surveillance of patients with UC has been evidenced. The probability of dysplasia detection has doubled [153].
High-resolution colonoscopy and dye chromoendoscopy in the case of SD-WLE were already recommended in 2015 as part of the surveillance for patients with UC (consensus statement of SCENIC – Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients) [154].
In further studies, HD-WLE proved to be as effective as DCE in detecting dysplastic lesions, with a shorter examination time (15.4 min vs. 24.6 min, p < 0.001) [141, 155, 156].
The next step was the development of virtual chromoendoscopy (VCE), in which the image, owing to digital processing, achieves the goals characteristic for typical dyeing – highlighting the vascular pattern as well as the mucosal pattern and lesions, and the lack of use of typical dyes reduces the cost and time of the examination. Endoscopic equipment manufacturers have developed various solutions – e.g. NBI (narrow band imaging, Olympus), iSCAN (Pentax), FICE (Fuji intelligent color enhancement, Fujifilm), LCI (linked-colour imaging, Fujifilm) and BLI (blue laser imaging, Fujifilm). In a randomised trial, DCE, VCE and HD-WLE were similarly effective in detecting colonic neoplasia in the surveillance of patients with UC [157]. The use of high-resolution colonoscopy with chromoendoscopy (HD-WLE + CE) significantly improved the detection of dysplastic lesions [141].
In 2019 (ACG guidelines), it was suggested to use virtual chromoendoscopy (NBI) or dye chromoendoscopy, as presumably equally effective [13]. This thesis was confirmed by a randomised multi-centre study in which no differences were found between VCE (NBI) and DCE in colonic dysplasia detection, but the withdrawal time with the NBI technique was significantly shorter (NBI 18.5 min vs. DCE 27 min; p < 0.001) [158]. Similar efficacy in dysplasia detection in comparison with DCE has also been demonstrated for i-SCAN and FICE techniques [13].
Currently, in the CRC surveillance of patients with IBD it is recommended to perform high-resolution colonoscopy with chromoendoscopy (virtual or using standard dyes) and to routinely take targeted biopsies from visible lesions.
According to the quality indicators proposed by the ESGE (European Society of Gastrointestinal Endoscopy), for surveillance colonoscopies in IBD the minimum percentage of colonoscopies using high definition endoscopy should be ≥ 90%, and those with the use of chromoendoscopy combined with targeted biopsies should be ≥ 70% [149].
It should be noted, however, that there have been identified subgroups of patients who benefit from taking additional specimens, including in a random manner (4 biopsies every 10 cm of the large intestine). These include, for example, high-risk patients with a history of colonic neoplasia, active inflammatory lesions, colonic strictures, lead pipe appearance of the colon, or coexisting PSC [159].
It is also believed that random biopsies should be performed also when, despite the existing recommendations, chromoendoscopy is not possible [160, 161].
Histopathological assessment of the collected biopsy specimens for the presence and grade of dysplasia in patients with UC is difficult, especially in the mucosa with inflammatory lesions, where, in addition to conventional low- and high-grade dysplasia, non-conventional – so-called indefinite or reactive – dysplasia is also diagnosed. It has been proven that significant differences exist in the assessment of dysplasia between different histopathologists. Because of this and the clinical implications associated with the diagnosis of dysplasia, it is recommended for dysplasia to be confirmed by two evaluating histopathologists [6, 162].
In recent years, there have been studies on the use of artificial intelligence methods in the diagnosis of colorectal polyps and lesions with suspected dysplasia, but so far there have been no studies in the setting of surveillance in patients with IBD. Progress in this area may well lead to further changes to the currently established rules of conduct [163].
27. We recommend starting surveillance for colorectal cancer 8 years after the diagnosis of ulcerative colitis and immediately after the diagnosis of primary sclerosing cholangitis.
The next colonoscopic examination should be scheduled on the basis of risk factor analysis.
Surveillance is not necessary in patients with isolated proctitis.
| Recommendation #27 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
Ulcerative colitis is the main risk factor for CRC [164]. In a patient with UC the risk of CRC is 1.7 times higher than in a person without UC. In patients with UC and CRC, the risk of cancer death is also increased 1.6 times in comparison with sporadic CRC. Colorectal cancer is also the main cause of death among IBD patients (responsible for approximately 10–15% of fatalities) [2, 165]. The cumulative risk of cancer is estimated at about 1%, 2%, and 5% after 10, 20 and > 20 years of disease duration, respectively [166]. In comparison with sporadic cancer, IBD-related CRC is associated with a poorer prognosis, lower histological differentiation, and more common proximal colon location of primary lesions [167]. Progression from chronic inflammation to neoplasia may occur multifocally; hence if dysplastic lesions are detected, a higher risk exists of the presence of dysplasia or synchronous (concurrent) or metachronous (developing after 6 months) cancer [141].
Cancer prognosis and surveillance is a challenge in patients with UC. Nevertheless, a decrease in CRC incidence is currently observed in this group. In the 1950s, the annual incidence rate was estimated at 4.29/1,000 patients, and in studies from the last decade it was 1.21–1.7/1,000 [166, 168]. The development of pharmacological and non-pharmacological methods of IBD treatment, the development of endoscopic techniques, and the introduction of surveillance principles are believed to have contributed to this change [151].
The proven risk factors for CRC in patients with UC are [151]:
disease duration (odds ratio (OR) = 4.74; the risk increases along with the increasing duration, especially when > 6–8 years),
extent of inflammatory lesions (OR = 2.43; increasing risk from pancolitis > left-sided colitis > proctitis),
activity of inflammation in macroscopic and microscopic assessment (OR = 2.62 and 1.98, respectively),
presence of strictures and inflammatory polyps (OR = 7.78 and 3.29, respectively),
coexistence of PSC (OR = 4.14),
CRC in family history depending on age and degree of relationship (OR = 2.62),
presence of dysplasia (OR = 10.7),
age at UC diagnosis < 16 years (OR = 1.27),
sex (slightly higher risk in males).
It is recommended that the first colonoscopic examination as part of oncological surveillance should be performed 8 years after disease diagnosis. The exception is UC patients with inflammatory lesions confined to the rectum (proctitis). They do not require endoscopic surveillance unless an increase in the extent of the disease is confirmed [151].
Other recommendations refer to patients with concomitant PSC, in whom surveillance for CRC should be started immediately after the diagnosis of PSC, regardless of the duration of UC. In patients with PSC, the overall risk of developing CRC reaches 31% and is four times higher than in patients without PSC [151].
The presence of CRC risk factors and the associated risk classification (low, intermediate, high) are determinant for the time of performing the next surveillance colonoscopy. The recommended principles of surveillance for CRC in UC patients (developed on the basis of evidence-based medicine and consecutive expert consensus statements: SCENIC 2015, ECCO 2019, BSG 2019, American Society of Colon and Rectal Surgeons – ASCRS 2021, ESGE 2021, AGA 2021) are presented in Table VI [3, 6, 18, 116, 154, 159, 169].
Table VI
Recommended principles of surveillance for colorectal cancer in ulcerative colitis patients
28. We recommend the use of mesalazine in the chemoprevention of colorectal cancer in patients with ulcerative colitis extending proximally from rectum.
(Quality of evidence: low; strength of recommendation: strong)
| Recommendation #28 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
The effect of 5-aminosalicylates on the development of CRC in UC patients has been extensively studied since the first favourable report on sulfasalazine use in 1994 [170]. The analysis of the evidence, taking into account its diversity and sometimes inconsistent results, supports the fact that the use of mesalazine reduces the risk of CRC in UC patients. Such an effect has not been demonstrated for sporadic cancer (in patients without UC) [171]. The mode of action of mesalazine is multidirectional; it has an effect on inflammatory and proliferative mechanisms, decreases nitric oxide activity and inhibits the Wnt/β-catenin pathway. It is unclear whether the chemopreventive effect is related to mucosal healing or specifically to the mechanism of action of the drug [172].
As shown in the CESAME study, mesalazine reduces the risk of CRC in patients with extensive UC (> 50% of the colon) and long duration of UC (> 10 years) [173]. Other studies have shown that other patients with UC also benefit from mesalazine use, except for those whose UC is confined to the rectum [18].
A meta-analysis of 31 observational studies confirmed the protective effect of mesalazine on the development of dysplasia and CRC in patients with UC. The risk of developing CRC was estimated to be reduced by 43% in patients treated with mesalazine (relative risk, RR = 0.57, 95% CI: 0.45–0.71). This effect is dose-dependent – it occurs when a daily dose of more than 1.2 g of mesalazine is used. No significant effect of sulfasalazine has been confirmed [174–176].
In view of the above, the expert consensus statements are consistent and recommend the use of mesalazine for chemoprevention of CRC in UC patients without a time limit [3, 18].
As the activity of colonic inflammation has been recognised as a risk factor for CRC, other drugs with proven efficacy in mucosal healing, i.e. thiopurines and targeted agents, mainly anti-TNF-α antibodies, were investigated for possible use in chemoprevention [177].
Studies with thiopurines have given conflicting results. Evidence has emerged that thiopurines reduce the risk of developing CRC in the course of long-term UC, but an increase in the risk of lymphoproliferative diseases, non-melanoma skin cancers, and urinary tract cancers has been observed [178–180].
A large meta-analysis of 95,397 patients with IBD confirmed a reduction in the risk of dysplasia, and CRC in the group treated with thiopurines, particularly with long-term disease (> 8 years), but this effect was not observed in subgroups of patients with extensive colon involvement and concomitant PSC [181].
In view of the risk of carcinogenesis, despite the evidence for a beneficial effect on the risk of CRC, thiopurines are not recommended for chemoprevention in expert consensus statements [3, 6].
Moreover, in recent years evidence has appeared that the use of anti-TNF-β agents may contribute to a reduction of CRC risk [182]. Two large retrospective cohort studies were published in 2021. In one of those studies, 246 CRC cases were observed in 32,403 UC patients treated with anti-TNF-α agents (annual incidence rate 1.24/1,000 patients; median follow-up 6.1 years). Anti-TNF-β therapy has been demonstrated to reduce the risk of CRC in a subgroup of patients with a long duration of UC (≥ 10 years), but this has not been proven in the entire study group [183]. In the second study (analysis of a database with 188,420 UC patients) anti-TNF-α therapy reduced the risk of developing CRC in the entire study group [184]. Interestingly, in another study the risk of CRC did not increase after mesalazine discontinuation in patients receiving anti-TNF-α therapy [185].
Anti-TNF-β therapy is not as yet recommended for the CRC chemoprevention in UC patients; the need for further analyses, especially prospective ones, is being discussed [183, 184].
The effect of other targeted agents currently used to treat UC (ustekinumab, vedolizumab, tofacitinib, upadacitinib, filgotinib, ozanimod) on the development of CRC is unknown. This requires studies and a longer follow-up period [186].
29. We recommend endoscopic surveillance in patients after radical endoscopic resection of polypoid or non-polypoid dysplastic lesions.
| Recommendation #29 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 8% | 92% | ||||
30. We recommend surgical treatment for visible dysplastic lesions that cannot be resected endoscopically, confirmed high-grade dysplasia or multifocal low-grade dysplasia diagnosed in the examination of random biopsies (without visible lesions), dysplasia in the flat mucosa surrounding visible dysplastic lesions, or if adenocarcinoma is found.
| Recommendation #30 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 8% | 92% | ||||
In colonoscopy in IBD patients, the Paris classification modified according to the SCENIC 2015 guidelines (Table VII) should be used to describe visible lesions suspected of dysplasia [154].
Table VII
Recommended terminology for describing macroscopic lesions found in colonoscopic surveillance for colorectal cancer in patients with inflammatory bowel disease (according to the Paris classification modified by the SCENIC guidelines) [154]
In recent years, owing to the development of high-resolution colonoscopy and virtual chromoendoscopy, a new scale has been developed for descriptive purposes – PICaSSO (the Paddington International Virtual ChromoendoScopy ScOre). It is used for the assessment and evaluation of both mucosal and vascular pattern. The data on its use seem to be promising. It has been shown to be consistent with the commonly used scales such as the Mayo score or UCEIS (Ulcerative Colitis Endoscopic Index of Severity) and to correlate well with histopathological assessment of lesions. It may prove to be a useful diagnostic tool for UC patients [157, 162].
Patient management varies depending on the type of macroscopic dysplastic lesion according to the above classification and histopathological examination. The clinical management algorithm (developed on the basis of evidence-based medicine and consecutive expert consensus statements: SCENIC 2015, ECCO 2017, ECCO- European Society of Gastrointestinal and Abdominal Radiology – ESGAR 2019, BSG 2019, ASCRS 2021, ESGE 2021) is presented in Figure 1 [3, 6, 18, 116, 149, 154, 159].
Figure 1
Suggested algorithm for the management of dysplasia detected in colorectal cancer surveillance colonoscopy in ulcerative colitis patient
HD-WLE + CE – high-definition white light endoscopy + chromoendoscopy, HGD – high-grade dysplasia, LGD – low-grade dysplasia.
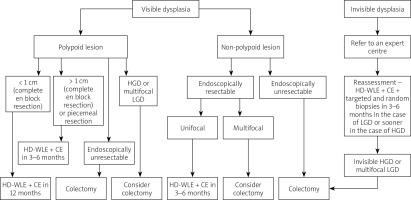
If colorectal adenocarcinoma is diagnosed, proctocolectomy or restorative proctocolectomy (with ileal pouch creation) is recommended. Nevertheless, in the absence of rectal involvement, colectomy could be exceptionally considered (decided on a case-by-case basis, only in selected patients) [10].
31. If indefinite dysplasia is found in the mucosa with active inflammatory lesions, we suggest intensification of pharmacological treatment and follow-up colonoscopy with biopsies within 3–12 months.
| Recommendation #31 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 25% | 75% | ||||
In a UC patient, the inflammation-dysplasia-cancer sequence of events leads to the development of CRC [186]. In the mucosa with active inflammatory lesions it is difficult to assess the presence of dysplasia on histopathological examination. In a situation where it is not possible to distinguish between “inflammation” and “dysplasia”, indefinite dysplasia, indeterminate dysplasia, non-conventional dysplasia or reactive atypia is diagnosed. Seven subtypes of such dysplasia are known [187].
The presence of indefinite dysplasia is associated with a higher risk of diagnosing classical dysplasia in the future. One study showed that when indefinite dysplasia was found (92% of diagnoses in targeted biopsies), low-grade dysplasia was present in 13% and high-grade dysplasia or cancer was present in 2% of patients in subsequent colonoscopy (median follow-up: 28 months) [188]. In another study dysplasia was diagnosed in up to 28% of patients with the presence of indefinite dysplasia in biopsy specimens randomly collected during the previous endoscopic examination. Furthermore, indefinite dysplasia was found in 21% of patients in whom conventional dysplasia was detected in the same intestinal segment and in 45% of patients with IBD and CRC [187].
There is emerging evidence that indefinite dysplasia may be associated with a higher risk of developing CRC than conventional dysplasia [189].
In an assessment of mucosa with a history or presence of inflammation, benefits from random sampling were demonstrated [190].
If indefinite/reactive dysplasia is diagnosed in visible lesions that cannot be endoscopically resected or in random biopsy specimens from inflamed mucosa, it should be attempted to obtain healing of such lesions by intensification of treatment and follow-up colonoscopy should be performed (the recommended modality is HD-WLE + CE with random and targeted biopsies). The time needed for the healing of inflammatory lesions may vary depending on their severity and the pharmacological treatment used [188, 189]. Therefore, it is considered that the follow-up examination should be performed within 3–12 months.
Patients in whom mucosal healing is not achieved and indefinite dysplasia is rediscovered require an individual approach, since the principles of optimal management are currently unknown due to the lack of relevant studies [3, 18, 154].
32. In patients after restorative proctocolectomy, especially with a history of colorectal cancer or with coexisting primary sclerosing cholangitis, we suggest conducting endoscopic surveillance of the ileal pouch. If adenocarcinoma, high-grade dysplasia or multifocal low-grade dysplasia is found in flat mucosa in biopsy specimens collected during pouch endoscopy, we suggest surgical treatment.
| Recommendation #32 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 8% | 92% | ||||
Patients who have undergone restorative proctocolectomy are at risk of developing dysplasia, especially in the case of CRC history or colonic dysplasia before surgery and PSC coexistence. In addition, a higher incidence of pouchitis is observed in patients with PSC [114, 115]. Although the quality of the evidence is low, the recommendations of experts/societies are in favour of regular endoscopic surveillance of the formed ileal pouch. Evidence is emerging that an increased risk of neoplasia in patients after restorative proctocolectomy may also apply to patients with chronic pouchitis or inflammation of the anorectal cuff (called cuffitis) and in the case of CRC in the family history. One study estimated that the cumulative risk of neoplasia development in the pouch is 0.9, 1.3, 1.9 and 5.1% at 5, 10, 15 and 25 years after pouch surgery, respectively [191]. Therefore, together with GETECCU (The Spanish Working Group on Crohn’s Disease and Ulcerative Colitis), we suggest that the first surveillance examination in patients with an ileal pouch should be performed 10 years after the diagnosis of UC, followed by examinations at 5-year intervals. However, in patients with risk factors such as colonic cancer or dysplasia before surgery and/or PSC, we suggest annual endoscopic surveillance. In the case of chronic pouchitis, cuffitis or CRC in the family history, endoscopic monitoring should be conducted every 1–3 years [114, 115].
Endoscopic assessment should include the pre-pouch ileum, the afferent and efferent loop, the distal pouch and the anorectal area with retroflexion and sampling. According to the 2021 ESGE guidelines, biopsies should be taken in a targeted manner from the visible lesions and randomly from the afferent ileal loop, efferent blind loop, the distal pouch and the anorectal cuff (at least 2 biopsies from each site) [159].
If adenocarcinoma, high-grade dysplasia or multifocal low-grade dysplasia is found in flat mucosa in biopsy sections collected during pouch endoscopy, surgical treatment is suggested. The diagnosis of low-grade unifocal dysplasia requires further supervision (monitoring every 3–6 months for 2 years and every 1 year thereafter is suggested), while the finding of indefinite dysplasia requires treatment of inflammation and endoscopic monitoring 3-6 months later [114, 115].
C. PSC – primary sclerosing cholangitis
33. In ulcerative colitis patients with suspected primary sclerosing cholangitis we recommend magnetic resonance cholangiopancreatography as the diagnostic method of first choice.
| Recommendation #33 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
Primary sclerosing cholangitis is an idiopathic cholestatic disease of the intra- and extrahepatic bile ducts, causing multifocal stenosis and pre-stenotic dilatation of the biliary tree. Over time, PSC can lead to liver fibrosis, cirrhosis and failure [1, 6, 192–194].
PSC can be diagnosed after ruling out other diseases leading to distortion and narrowing of bile ducts, mainly cancers of bile ducts, the ampulla of Vater or pancreas, IgG4 cholangiopathy, chronic pancreatitis, ischaemic bile duct injury, hepatic hilar lymphadenopathy, recurrent bacterial cholangitis related to cholelithiasis and opportunistic infections in acquired immunodeficiency syndrome (AIDS) [192, 193].
The annual incidence of PSC is estimated at 0.91–1.3/100,000 (0.15/100,000 for small-duct PSC), and the prevalence is about 16 cases of PSC per 100,000 people. PSC occurs in 8% of patients with UC and is usually diagnosed at 30–40 years of age. Approximately 60–70% of patients with PSC and UC are male. In PSC, 58% of patients with IBD have lesions in the extra- and intrahepatic bile ducts, in 40% of patients with PSC the lesions affect only the intrahepatic ducts, and in 2% the lesions are limited to the extrahepatic ducts. In 8% of patients with PSC a variant known as small-duct PSC is present [192–194].
Typical PSC symptoms are pruritus, chronic fatigue, discomfort in the right upper quadrant of the abdomen, and recurrent febrile states. Jaundice, occurring in about 50% of patients at diagnosis, is a symptom of advanced disease. The mean time from the diagnosis to death or liver transplantation is 13–20 years. The first biochemical symptom of PSC is elevated liver enzymes, especially of the cholestatic type (alkaline phosphatase, ALP; γ-glutamyltransferase, GGT) [192–194]. In a patient with UC, PSC should be suspected if increased levels of ALP and/or GGT are noted within 1–3 months, apart from typical symptoms. Such a patient requires diagnostic evaluation for PSC [192, 194].
In the initial stages of the disease, cholestatic enzyme elevations may be temporary, and thus it is good practice in patients with UC to periodically monitor ALP and GGT (at least every 12 months). Moderately elevated transaminases (2–3× upper limit of normal) may be found in laboratory tests in PSC, but a significant increase (ALT > 5× upper limit of normal) raises the suspicion of coexistence of autoimmune hepatitis (AIH). Hyperbilirubinaemia is usually a consequence of the dominant strictures or appears during the period of cirrhosis. The titres of pANCA, anti-nuclear antibodies (ANA), anti-smooth muscle antibodies (ASMA) and IgMs or IgGs, which may be elevated in more than 50% of patients, are not specific for PSC, and thus have little diagnostic relevance, although they may be useful in differential diagnosis. A marker of high value for differentiation from primary biliary cholangitis (PBC) is anti-mitochondrial antibodies (AMA), which are positive in only < 5% of patients with PSC, along with PBC-specific ANA (sp100, gp210). Because of the similarity of cholangiographic images in PSC and IgG4 cholangiopathy, serum IgG4 antibody levels should be measured at least once in each PSC patient. Moderately elevated IgG4 levels occur in about 20% of patients with PSC and, according to some authors, may be associated with faster disease progression [192, 195–198].
Owing to its availability, ultrasound is often the first imaging test performed in patients with PSC. Ultrasound may reveal gallbladder stones or polyps and, less frequently, segmental dilatation of the bile ducts; nevertheless, its value in the diagnosis of PSC is low. Normal appearance of the bile ducts on ultrasound does not rule out PSC.
The basis for the diagnosis of PSC is magnetic resonance cholangiopancreatography (MRCP), which typically shows alternating dilatations and strictures of the bile ducts (the “beads-on-a-string” sign) and the “pruned-tree” appearance in late stages of the disease. The sensitivity and specificity of MRCP (86% and 94%, respectively) in the diagnosis of PSC is comparable to endoscopic retrograde cholangiopancreatography (ERCP), previously considered the gold standard in the diagnosis of PSC. Currently, the role of ERCP in PSC is mainly the dilatation of dominant bile duct strictures and the collection of material for microscopic examination if bile duct cancer or IgG4 cholangiopathy is suspected. Performance of ERCP may also be considered when there is a strong suspicion of PSC and MRCP is contraindicated, or the biliary tree appearance is inconclusive [6, 193–199].
Liver biopsy enables the diagnosis of small bile duct PSC, in which lesions are limited to the interlobular ducts and the MRCP appearance of the intra- and extrahepatic bile ducts is normal. Liver biopsy is also helpful in diagnosing the PSC/AIH overlap syndrome and other causes of unexplained cholestasis [196–201].
34. In patients with newly diagnosed primary sclerosing cholangitis we recommend colonoscopy with biopsies to search for inflammatory bowel disease, especially ulcerative colitis.
| Recommendation #34 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
Inflammatory bowel disease is diagnosed in 62–83% of PSC patients in population studies in Northern Europe and in a smaller percentage in southern European and Asian countries. It is believed that these data may be underestimated due to the often oligosymptomatic course of bowel inflammation in patients with PSC [1, 6, 192, 194].
The diagnosis of IBD usually precedes the diagnosis of PSC, but bowel disease can also manifest itself after a diagnosis of PSC and even after liver transplant. In patients with IBD and PSC, the risk of CRC increases significantly in comparison with patients without PSC. This is especially true in patients with UC, in whom it is 56% higher than in patients with Crohn’s disease. Patients with PSC without coexisting IBD also have a higher risk of CRC, which in one study was estimated to be 2% in a 10-year follow-up. In another study, 3 cases of CRC were diagnosed among 200 patients with PSC without IBD [1, 6, 192, 202].
In view of these facts, in patients with newly diagnosed PSC it is recommended to perform ileocolonoscopy, even in the absence of IBD symptoms, and to collect biopsy specimens despite the absence of macroscopic lesions. In addition, it is believed (despite the lack of solid evidence) that in patients with PSC without inflammatory lesions in the first colonoscopy it is useful to repeat this examination at 5-year intervals if they remain asymptomatic or more often if symptoms suggestive of IBD occur [6, 202–204].
35. We recommend cancer surveillance in patients with ulcerative colitis and primary sclerosing cholangitis, in view of the increased risk of colorectal cancer, biliary duct and gallbladder cancer, and hepatocellular carcinoma if liver cirrhosis develops.
| Recommendation #35 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
Patients with PSC have an increased risk of CRC and of biliary duct and gallbladder cancer. In addition, in the situation of liver disease progression and the development of cirrhosis, the risk of hepatocellular carcinoma also increases [6, 203–213].
Biliary and colorectal cancers are considered the leading causes of death in patients with PSC (32% and 8%, respectively). The risk of CRC and the principles of surveillance in patients with PSC are described in a separate recommendation. The risk of biliary duct cancer in patients with PSC in the typical form (large-duct PSC) is about 400 times higher than in the general population. The annual, ten-year and thirty-year risk of developing biliary duct cancer is estimated at about 2%, 6–11% and 20%, respectively. Notably, 30% of biliary tract cancer diagnoses are made in the first year after PSC diagnosis [6, 203–213].
Risk factors for biliary tract cancer in PSC are age (incidence 1.2/100 patient-years in persons < 20 years of age versus 21/100 patient-years in persons > 60 years of age), male sex, and the presence of UC. No increase in the risk of biliary tract cancer is observed in the subgroup of patients with small duct PSC and in paediatric patients [203, 204].
It has been proven in population studies that regular surveillance for early biliary duct cancer increases the five-year survival rate. Surveillance includes non-invasive imaging examinations – ultrasound, magnetic resonance imaging (MRCP ± contrast-enhanced MRI), less often (due to exposure to ionising radiation) computed tomography or ERCP. In addition, CA 19-9 levels are used in cancer surveillance, and not a single measurement, but rather the increase of the levels of this marker in subsequent tests is of diagnostic significance [203, 204, 208].
The sensitivity and specificity of these methods are not optimal (sensitivity and specificity: ultrasound 57% and 94%, MR/MRCP 89% and 75%, respectively). These values are improved when biliary tract imaging is combined with CA 19-9 testing. CA 19-9 tests may give false positive results in a PSC patient with severe cholestasis and especially cholangitis. On the other hand, CA 19-9 has no diagnostic significance in 5–10% of the population, for genetic reasons (“non-secretors”) [203, 204, 206].
The risk of gallbladder cancer in patients with PSC is estimated at 2%. The incidence of gallbladder cancer in patients with PSC is estimated as 1.6/100 patient-years [209].
The precancerous condition is adenomatous gallbladder polyp present in 10–17% of patients with PSC. In surveillance, gallbladder imaging methods are used, especially ultrasound – its sensitivity and specificity in the detection of gallbladder polyps are 84–96%. The size of the polyp is important for the risk of developing gallbladder cancer. In one study in patients with PSC, polyps < 8 mm in diameter on histopathological examination (after cholecystectomy) did not contain dysplasia, and polyps < 12 mm did not contain cancer lesions [203, 204, 209].
On the basis of this and other studies, an overall conclusion was drawn that the size of the polyp ≥ 8 mm in ultrasound enables the diagnosis of neoplasia with a sensitivity of 96% and a specificity of 53%. It should be noted, however, that cancerous transformation was observed in polyps < 10 mm or even 6 mm in size in patients with PSC.
If a polyp is detected in the gallbladder in a patient with PSC, it is also important to assess the risk associated with cholecystectomy, which increases with the progression of liver disease [203, 204, 209].
The risk of hepatocellular carcinoma in patients with PSC increases with disease progression to cirrhosis and is no greater than with cirrhosis from other causes. Therefore, the principles of supervision of a patient with PSC-related cirrhosis do not differ from the principles of supervision in cirrhosis from other causes [203, 204].
The diagnosis of cirrhosis in a patient with PSC may influence therapeutic management decisions, e.g. concerning planned colectomy or bile duct cancer treatment. Liver failure and complications of portal hypertension are important causes of death in patients with PSC. Therefore, in the surveillance of patients with PSC, non-invasive methods of assessing liver fibrosis (e.g. elastography) are worth considering, especially as the result of the FibroScan liver tissue stiffness test > 25 kPa with a platelet count of < 110,000/mm3 should prompt endoscopic assessment for oesophageal varices.
Due to the progressive course of PSC, patients should be subject to regular elastography monitoring, at least at annual intervals [203, 204, 206, 208].
The principles of cancer surveillance in PSC, developed on the basis of literature and expert consensus statements, are presented in Figure 2.
Figure 2
Suggested cancer surveillance algorithms in a patient with primary sclerosing cholangitis
PSC – primary sclerosing cholangitis, UC – ulcerative colitis, IBD – inflammatory bowel disease, US – abdominal ultrasound, MRI – magnetic resonance imaging, MRCP – magnetic resonance cholangiopancreatography, CT – computed tomography, ERCP – endoscopic retrograde cholangiopancreatography, AFP – α-fetoprotein.

36. We do not have sufficient evidence to recommend routine use of ursodeoxycholic acid in patients with newly diagnosed primary sclerosing cholangitis to slow the progression of liver damage and prevent colorectal and biliary cancer.
(Quality of evidence: moderate; strength of recommendation: strong)
| Recommendation #36 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 17% | 17% | 66% | |||
At the present stage of knowledge, the causal treatment of PSC is unknown. Ursodeoxycholic acid (UDCA) has commonly been used for about 30 years due to its potentially beneficial biological effects (hepatoprotection, inhibition of apoptosis, protection of cholangiocytes against the harmful effects of hydrophobic bile acids, increase in bile acid secretion, anti-inflammatory effect) and a good safety profile when administered at a dose of 13–20 mg/kg/day [194, 203, 204, 214–228].
The assessment of UDCA efficacy in the treatment of PSC is inconclusive. The drug may improve laboratory parameters when used at a daily dose of 15–20 mg/kg, but in monotherapy it has no significant effect on slowing disease progression. In meta-analyses of studies (including approximately 1,000 patients), despite improvements in biochemical tests, there was no evidence of significant benefits of UDCA in terms of risk of death and time to liver transplantation, histopathological or cholangiographic improvement, and the risk of developing biliary cancer. There was also no evidence of a significant reduction in the risk of CRC in PSC patients treated with UDCA. In addition, it has been found that high doses of UDCA (28–30 mg/kg/day) may be harmful (increased risk of death or occurrence of oesophageal varices, shortened time to liver transplantation). Also in publications of series of small-duct PSC cases treated with UDCA, despite the observed improvement in biochemical tests, no significant benefit was confirmed in terms of disease progression, occurrence of complications, or risk of death or transplantation [214–226].
At present, routine use of UDCA in patients with newly diagnosed PSC is not recommended. However, in one study of patients treated with UDCA, its discontinuation after a three-month follow-up was associated with increased pruritus and elevated levels of ALP, GGT, transaminases and bilirubin [203, 204].
37. We recommend carrying out the treatment of ulcerative colitis in patients with primary sclerosing cholangitis in accordance with the general principles. When selecting the most appropriate therapeutic options, the stage of liver injury and the particularities of the course of bowel disease in primary sclerosing cholangitis should be taken into account.
(Quality of evidence: low; strength of recommendation: strong)
| Recommendation #37 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
Ulcerative colitis coexisting with PSC is usually oligosymptomatic, with endoscopic lesions located mainly in the right half of the colon, with retrograde involvement of the terminal ileum and lesser severity of the lesions present in the rectum [1, 6, 192, 203, 204, 229].
Medicines used in the treatment of UC, i.e. steroids (prednisone, budesonide), immunosuppressants (azathioprine, cyclosporine) or biological agents (anti-TNF-α, vedolizumab), have not been proven to have a negative or positive influence on the natural history of PSC, although a decrease in ALP activity was observed in patients treated with adalimumab or vedolizumab [214, 229, 230].
For other targeted agents (ustekinumab, tofacitinib, upadacitinib, filgotinib, ozanimod) used to treat UC, there are no data on their use in PSC.
A good practice in a patient with PSC undergoing immunosuppressive or biological therapy for UC is to consider antibiotic prophylaxis before ERCP [199, 203, 204].
The stage of liver disease may impact therapeutic decisions in a patient with UC. An example is budesonide – if cirrhosis develops, this medicine loses its advantage associated with the first-pass effect and can then cause undesirable systemic effects [203, 204, 230].
During the period of liver failure, patients with PSC are considered to be candidates for liver transplantation. Remission of UC and cigarette smoking cessation improve the outcomes of transplantation treatment.
The evolution of UC after liver transplantation is unpredictable; inflammatory activity may increase or decrease while the patient remains in remission. According to multivariate analysis, the use of ciclosporin as part of immunosuppressive therapy has a beneficial effect on the course of UC after transplantation. There are known cases of de novo UC after liver transplantation performed for PSC (the risk is 10–11%) [230, 231].
Recurrence of PSC in the transplanted liver affects 10-40% of patients, and active UC is one of the risk factors for PSC recurrence. It is unclear whether performing colectomy before or during transplantation reduces the risk of PSC recurrence. Previous data on the protective importance of colectomy with a 20% reduction in the risk of PSC recurrence were not confirmed in a recently published study of 531 patients from 6 transplant centres in Europe and North America, in which colectomy did not reduce the risk of PSC recurrence. That study has confirmed that PSC recurrence is associated with IBD activity [229, 231, 232].
If a decision is made to perform colectomy, it is worth considering the fact that the creation of end-ileostomy is associated with better results of liver transplantation than ileal pouch surgery. Patients after liver transplantation due to PSC are still subject to annual endoscopic surveillance for UC in view of their risk of developing CRC (the expected annual incidence of CRC in patients treated with immunosuppressants is 1%) [203, 204].
D. Pregnancy
38. During the period of remission of ulcerative colitis, fertility in women is not impaired. In the active phase of ulcerative colitis, both female and male fertility may be reduced. Past abdominal surgery in women may make it more difficult to get pregnant.
| Recommendation #38 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
39. The use of sulfasalazine in men may induce a reversible decrease in sperm count and motility. There is no conclusive evidence of a negative impact of steroids, mesalazine, thiopurines, anti-TNF agents, ustekinumab and vedolizumab on the fertility of patients with ulcerative colitis and an increased risk of developing congenital defects in the offspring.
| Recommendation #39 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 16% | 84% | ||||
40. In the case of pregnancy in a woman in clinical remission of ulcerative colitis, we suggest continuation of the existing treatment (except methotrexate). The risk of pregnancy failure associated with exacerbation of the disease is much greater than the potential risk of adverse effects of treatment.
(Quality of evidence: low; strength of recommendation: weak)
| Recommendation #40 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
There is no evidence for reduced fertility in patients with UC in remission [14, 233–236]. Lower fertility, described in many analyses, results mainly from the decision of patients who are afraid of a complicated course of pregnancy, the risk of foetal defects, and the risk of IBD development in their offspring. However, there are data showing that an active uncontrolled form of UC, with high systemic activity of the disease process, may negatively affect both female and male fertility [14, 233–236]. This may result from the direct influence of inflammatory mediators on a number of processes related to fertility (e.g. ovulation disorders, erectile dysfunction, impact on sperm quality). There is also some evidence that major abdominal and pelvic surgery may increase the risk of problems with getting pregnant [14, 233, 244]. On the other hand, in men pelvic procedures may result in erectile dysfunction and ejaculation disorders. It should be stressed, however, that the relevant data are very limited. As for the medicines, it has only been demonstrated that sulfasalazine may have a reversible negative impact on sperm quality in men [14, 234–236]. A medicine absolutely contraindicated both in the periconception period and during pregnancy and lactation is methotrexate, although it is currently less and less used in UC due to doubts about its efficacy in this indication [14, 233, 234, 237]. Methotrexate (especially in the case of foetal exposure in the first trimester of pregnancy) has been evidenced to increase the risk of miscarriage and of a number of congenital defects. Therefore, it is recommended to discontinue methotrexate (in both women and men) approximately 6 months before the planned procreation. The remaining medicines have no negative effect on fertility [14, 233, 234].
The key condition for maintaining reproductive capacity and for the normal course of pregnancy and delivery is obtaining remission of UC. Any possible risks related to the treatment are significantly lower than those resulting from the uncontrolled course of UC. Therefore, in most cases of pregnancy the treatment used so far, which ensured remission of the disease (except methotrexate), should be continued [14, 233, 234]. In many registries (e.g. PIANO) and numerous observational studies, no increase in the rate of pregnancy loss or congenital defects in the offspring was observed when mothers were treated with aminosalicylates, thiopurines or anti-TNF agents [14, 233, 234, 238]. The existing data for vedolizumab and ustekinumab also do not provide any relevant conclusive information that would raise concern. However, in the light of current knowledge, Janus kinase inhibitors and ozanimod should not be used during pregnancy [14, 234].
Anti-TNF agents cross the placenta, and the greatest placental transfer is observed in the third trimester of pregnancy. It has been demonstrated in many analyses that these antibodies are detectable in children of mothers with IBD undergoing biological therapy during pregnancy, up to 6 months of age. Therefore, if possible (i.e. in the case of long-term remission of UC), discontinuation of therapy at the beginning of the third trimester of pregnancy may be considered to limit foetal exposure to the medicine [14, 233, 234, 239]. However, this practice should not be used routinely and treatment may be continued throughout the pregnancy. On the other hand, in children of mothers with IBD treated with anti-TNF antibodies during pregnancy (this probably also applies to other biologics) administration of live vaccines should be deferred (it is usually suggested that they can be administered only after 6–12 months of age or after the biological agent becomes undetectable in the child’s blood) [14, 233, 234, 239].
Also during lactation it is possible to continue UC therapy. Aminosalicylates, thiopurines, steroids and anti-TNF drugs are considered safe in this regard [14, 233, 234, 239]. Data on ustekinumab and vedolizumab are scarce, but observations so far have not yielded any alarming conclusions. The medicine contraindicated during lactation is methotrexate, and also the use of metronidazole and Janus kinase inhibitors as well as ozanimod should be avoided [14, 234].
41. In the case of a new diagnosis of ulcerative colitis or disease flare during pregnancy, we suggest treatment with mesalazine, steroids and/or anti-TNF agents. The choice of treatment depends mainly on the clinical condition of the pregnant woman. There are less data on vedolizumab and ustekinumab, but the evidence to date indicates their good safety profile, while the use of Janus kinase inhibitors and ozanimod is not recommended.
(Quality of evidence: low; strength of recommendation: weak)
| Recommendation #41 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 8% | 92% | ||||
The rules for the treatment of UC exacerbation in pregnancy are generally consistent with the standard rules of disease management. However, the assessment of disease activity should be based mainly on non-invasive parameters [14, 233, 234, 239]. Among laboratory tests, the calprotectin stool test is crucial. If it is absolutely necessary to perform gastroscopy, sigmoidoscopy or even ERCP, there are no contraindications for their use, but in the case of ERCP every effort should be made to minimise the exposure of mother and child to ionising radiation. The imaging modalities of choice are ultrasound and magnetic resonance imaging [14, 233, 234, 239].
The medicines most commonly used for the treatment of exacerbations are systemic steroids [14, 233, 234, 239]. There are some reports that when used in the first trimester of pregnancy, these medicines may slightly increase the risk of cleft palate in the foetus, but these data are of very poor quality [14, 233, 234]. One of the largest analyses in this area, that included a group of over 51,000 pregnancies, did not confirm this observation [240]. The most recent analysis, concerning data from the PIANO registry, suggests that steroid use during pregnancy may increase the risk of preterm birth, foetal growth disorders or the need for hospitalisation of the neonate in an intensive care unit, or that there may be an increased risk of infections between 9 and 12 months of age in children of mothers treated with steroids [241]. Therefore, some experts suggest the need to limit routine steroid use in connection with IBD exacerbations in pregnancy in favour of other drugs (mainly biological drugs, especially anti-TNFs) [234, 241]. However, this issue requires further studies. On the other hand, there are no clear safety signals regarding the use of oral budesonide in the treatment of UC [233, 234, 239].
Anti-TNF agents are considered safe in the treatment of UC exacerbations in pregnancy [233, 234, 239]. Data on other biologicals (vedolizumab, ustekinumab) are more limited, but there is currently no evidence of their negative effects on pregnancy or foetal development [233, 234, 239]. Janus kinase inhibitors and ozanimod are contraindicated in pregnancy in the light of current knowledge [234]. If antibiotic therapy is necessary, treatment with metronidazole and ciprofloxacin should be avoided – especially in the first trimester of pregnancy, and also during lactation [233, 234, 239]. However, the indications for surgical treatment in women with UC during pregnancy do not differ significantly from the indications in non-pregnant women [233, 234, 239].
42. In patients with an ileostomy, there are no gastroenterological contraindications for vaginal delivery. In patients after restorative proctocolectomy, the decision to deliver by caesarean section should be made on a case-by-case basis, taking into account obstetric indications, after consultation with a gastroenterologist and surgeon.
| Recommendation #42 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 42% | 58% | ||||
We do not have any data showing that the mode of delivery in a pregnant woman with UC would negatively affect the further course of IBD [233, 234]. The decision to deliver naturally or by caesarean section in a patient in remission should be based on obstetric considerations. An indication for caesarean section may be active perianal lesions, rarely encountered in UC. Since sometimes there is an increased risk of perineal tissue injury during natural childbirth, the decision on how to complete the delivery in a woman after IPAA should be made on a case-by-case basis, taking into account this risk [233, 234]. Similarly, the option of delivery by caesarean section should be discussed with a pregnant woman with UC if there is a high likelihood that she may require surgical treatment of IBD after pregnancy [233, 234].
E. Osteoporosis and osteopenia
43. Patients with osteopenia and those treated with systemic steroids should receive calcium and vitamin D products. If osteoporosis is diagnosed, we suggest treatment with bisphosphonates.
(Quality of evidence: moderate; strength of recommendation: weak)
| Recommendation #43 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
Osteopenia and osteoporosis are among the most common extraintestinal complications of UC. The contributing factors are inflammatory activity of the disease, vitamin D or calcium deficiencies, as well as deficiencies of other micro- and macroelements, malnutrition, physical inactivity [3, 6, 14, 194]. These phenomena may also be the result of treatment, mainly steroid therapy. Therefore, these diseases should be actively sought in every patient with active UC, in people with a long history of the disease, in the presence of additional risk factors for reduced bone mineral density and in patients undergoing steroid therapy (especially if the treatment is conducted for > 3 months) [6, 14, 194, 240–242]. The diagnostic examination of choice is bone densitometry, measured at the level of femoral neck and/or lumbar spine using DEXA (dual energy X-ray absorptiometry). Persons exposed to steroids for a long time and patients with osteopenia should be administered calcium 500–1000 mg/day and vitamin D 800–1000 IU/day (higher doses of vitamin D are specified by some recommendations) [3, 14, 194, 242–244]. Physical activity should be advised to all patients; tobacco smoking is contraindicated. However, the crucial factor is the optimal treatment of the underlying disease [14, 194, 242–244]. In the event of pathological fractures in people with osteoporosis, bisphosphonate therapy should be initiated. The use of bisphosphonates for the prevention of bone fractures in people with reduced bone mineral density is not recommended by the ECCO experts. It should be stressed, however, that research studies in this area are ongoing. The risk of developing complications of osteoporosis in patients with reduced bone mineral density should therefore be individually assessed and the appropriate treatment should be adjusted accordingly [3, 14, 194, 242].
F. Nutrition therapy
44. Normal nutritional status improves the outcomes of treatment for ulcerative colitis.
(Quality of evidence: high; strength of recommendation: strong)
| Recommendation #44 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
Malnutrition is a common complication of UC. Malnutrition is caused, for example, by increased catabolism in patients with an active uncontrolled disease, by nutritional deficiencies, and also by the treatment used [3, 14, 111, 245]. Therefore, nutritional status should be assessed in each patient using the commonly available scales. Normal nutritional status improves the long-term prognosis of UC. It also makes it possible to optimise the outcomes of pharmacological therapy and has an impact on the efficacy and safety of surgical treatment [3, 14, 111]. Given that the diet is an important environmental factor in the pathogenesis of IBD, and in view of the high interest of patients in the role of diet, an important element of a holistic approach to the care of IBD patients is the possibility of obtaining a professional consultation with a clinical dietitian who has appropriate qualifications and experience [14, 245].
G. Anaemia
45. If anaemia is detected, its type should be determined and then adequate treatment should be provided.
(Quality of evidence: moderate; strength of recommendation: strong)
| Recommendation #45 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
Anaemia is estimated to affect about 1/3 of IBD patients. The cause of anaemia is usually multifactorial and includes iron deficiency as well as anaemia of chronic diseases [3, 6, 14, 246]. Sometimes the aetiology also includes a deficiency of vitamin B12, less often folic acid. Anaemia significantly affects the course of UC and, in addition to its typical symptoms (such as fatigue or tachycardia), may interfere with tissue healing, reducing the efficacy of classical pharmacological therapy [3, 6, 14, 246].
Haemoglobin testing is necessary in each patient with UC; it is also helpful to assess the ferritin level, and possibly transferrin saturation. Anaemia is defined as haemoglobin < 12 g/dl in women (in pregnant women < 11 g/dl) or < 13 g/dl in men [6, 14, 246, 247]. In a patient with IBD without active inflammation, iron deficiency can usually be diagnosed if the ferritin level is < 30 µg/l (or transferrin saturation is < 20%), and in a patient with active inflammation it can be diagnosed if the ferritin level is less than 100 µg/l [6, 14, 246, 247]. Other tests (e.g. vitamin B12 level testing) should be performed as needed.
Treatment of anaemia in UC usually includes intensification of anti-inflammatory therapy (in patients with an active disease) and iron supplementation. If haemoglobin is below 10 g/dl, intravenous iron administration to correct its deficiencies is necessary [6, 14, 247]. The most commonly used formulations include iron isomaltoside or ferric carboxymaltose. The total dose of iron to be administered can be calculated using the Ganzoni formula or, in a simplified way, using the haemoglobin value and the patient’s weight (the usual dose is 1000–2000 mg). In a patient without active inflammation, oral iron supplementation at a dose not exceeding 100 mg/day is allowable in the case of mild anaemia with haemoglobin above 10 g/dl [14, 246, 247]. However, if oral iron dosing is not tolerated or haemoglobin is < 10 g/dl, as well as in the case of active disease, intravenous infusion of iron-containing products is necessary, which is considered the most optimal form of treatment for iron deficiency anaemia. An increase in haemoglobin by at least 2 g/dl within approximately 4 weeks is considered a response to treatment [14, 246, 247]. In the absence of a response, it is necessary to review the existing treatment; erythropoietin administration with intravenous iron supply may be considered in some patients. Blood transfusion is indicated only in patients with severe symptomatic anaemia (usually haemoglobin < 7 g/dl) [14, 246, 247].
H. Skin lesions
46. In the case of skin lesions of pyoderma gangrenosum or erythema nodosum type, systemic steroids should be used, and in the event of their failure anti-TNF therapy is suggested. The efficacy of other medicines in this indication is less well understood.
(Quality of evidence: moderate; strength of recommendation: weak)
| Recommendation #46 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 42% | 58% | ||||
The most common skin lesions that may accompany UC include erythema nodosum and pyoderma gangrenosum [6, 14, 194, 248, 249]. The diagnosis of these extraintestinal manifestations of UC should be based mainly on clinical presentation. In ambiguous cases with an atypical course, the best diagnostic method is histopathological evaluation of skin lesion biopsy specimens [14, 248, 249].
Erythema nodosum is the presence of painful subcutaneous tissue lumps or nodules with a diameter of 1–5 cm, usually red-violet in colour, appearing most often on the extensor parts of the lower legs. The incidence of erythema nodosum is closely correlated with the clinical activity of UC, and therefore its treatment should involve intensification of UC therapy. Systemic steroids are the treatment of choice. If steroid therapy fails or in recurrent lesions immunosuppressive therapy should be instituted; anti-TNF antibodies have also proven to be effective [14, 248–250]. The efficacy of other targeted therapies, including oral small molecule drugs, has been less well studied [248].
Pyoderma gangrenosum may affect any area of the skin. Most often, however, this dermatosis develops on the shin, while in patients with an enterostomy it is observed in the vicinity of the stoma orifice [6, 14, 194, 248, 249]. Initially, pyoderma gangrenosum is manifested as isolated inflammatory nodules similar to boils or pustules. These are followed by dermal necrosis leading to the formation of painful ulcers often covered by necrotic scabs. Pyoderma gangrenosum may also occur in patients in clinical remission of UC. The treatment of first choice for this dermal manifestation of UC is systemic steroids. In the absence of a timely response to this treatment, infliximab or adalimumab is the drug of choice [6, 14, 194, 248–250]. Alternative medicines are calcineurin inhibitors (ciclosporin, tacrolimus). The efficacy of other targeted therapies, including oral small molecule drugs, has been less well studied [14, 249]. Stoma closure should be considered, if possible, if pyoderma gangrenosum skin lesions develop in the area of the stoma orifice [14, 249]. If gastrointestinal tract continuity cannot be restored (in the event of removal or complete dysfunction of the sphincter apparatus), healing of the lesions around the stoma should be obtained and the stoma should be retained, or stoma relocation procedure may be considered, which may be possible after introducing conservative treatment and confirmation of its efficacy. Stoma relocation in a patient with severe pyoderma gangrenosum lesions may not only significantly impair ostomy wound healing, but also pose the risk of development of skin lesion around the newly formed stoma [194, 248, 249].
I. Arthropathy associated with UC
47. In the case of arthropathy associated with ulcerative colitis, treatment of the underlying disease should be intensified in the first place. In patients with peripheral joint lesions, additional sulfasalazine treatment, short-term treatment with non-steroidal anti-inflammatory drugs, topical steroids and physiotherapy may be helpful. If axial lesions are present, we suggest anti-TNF therapy in addition to physiotherapy. The efficacy of other therapeutic agents in this indication is less well understood.
(Quality of evidence: moderate; strength of recommendation: weak)
| Recommendation #47 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
Arthropathies associated with UC can be of peripheral and axial type. Peripheral arthropathy usually involves large joints (subtype 1) [6, 14, 194, 248]. A distinctive feature of this subtype is the asymmetry of lesions. This arthropathy subtype is usually acute and correlates with UC activity. The less common subtype 2 of peripheral arthropathy involves the small joints of the hand and is not dependent on UC activity. In both cases, the diagnosis is based on the clinical presentation (joint pain) and physical examination (painful swelling of joint areas in the case of arthritis) [6, 14, 194, 248]. Treatment of peripheral arthropathy should include intensification of UC therapy (steroids, immunosuppression, anti-TNFs, while the efficacy of other targeted agents, including oral small molecule drugs, has been less well studied) [6, 14, 194, 248, 251–253]. Short-term use of NSAIDs, preferably those from the cyclooxygenase-2 inhibitor class (coxibs), is acceptable. Local steroid injections along with physiotherapy are also recommended in selected cases. Sulfasalazine may be used, especially in peripheral arthritis of subtype 1 [6, 14, 194, 248, 251].
Axial arthropathy consists of sacroiliac arthritis and spondylitis. Its typical symptoms include chronic back pain partially relieved by physical exercise, and morning stiffness. The most recommended diagnostic procedure is magnetic resonance imaging of the osteoarticular system [6, 14, 194]. Axial arthritis in patients with UC can be treated with NSAIDs, but they should be used at minimal effective doses for the shortest possible time and selective cyclooxygenase-2 inhibitors are preferred. Physiotherapy also plays an important role. No satisfactory efficacy has been observed for such medicines as thiopurines, sulfasalazine or steroids. Since the use of NSAIDs should be kept to a minimum in patients with IBD, an alternative with proven efficacy is provided by anti-TNF agents. The efficacy of other targeted therapies, including oral small molecule drugs, has been less well studied [6, 14, 194, 248, 251–253].
J. Vaccination
48. In each patient with newly diagnosed ulcerative colitis, a full course of preventive vaccinations should be carried out or completed, if possible.
| Recommendation #48 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 100% | |||||
Both the medicines used to treat UC and sometimes the disease itself can increase the risk of several infectious diseases. Another challenge in patients with UC who are in an immunocompromised state may be the atypical course and treatment refractoriness of infectious diseases [14, 254–256].
Therefore, at the time UC is diagnosed, a thorough history of infectious diseases and preventive vaccination should be collected. It is particularly important to make sure that an adult patient with UC has received all vaccinations according to the mandatory immunisation schedule before the age of 18 years [14].
Assessment of the immunisation status against individual infectious diseases before the initiation of immunosuppressive therapy gives the opportunity to effectively and safely complete the course of vaccination. Immunosuppressed patients are defined as patients receiving steroids at daily doses of more than 20 mg prednisone equivalent for > 2 weeks and patients treated with effective doses of thiopurines, methotrexate, biological agents, and small molecule drugs, as well as malnourished patients [14, 254, 255]. In such cases live vaccines may be used no later than 3 weeks before the start of the above therapies and not earlier than 3 months after their completion. Live vaccines include tuberculosis (BCG) vaccine, measles, mumps and rubella (MMR) vaccine, chickenpox vaccine, oral polio vaccine, yellow fever vaccine, and oral rotavirus vaccine. Inactivated vaccines can be used safely in immunocompromised patients, but the efficacy of immunisation may be lower than in healthy individuals [14, 254–256].
In any adult person not immunised against a particular infectious disease (either by protective vaccination or by recovery from the infectious disease resulting in permanent immunity), at least the following additional vaccinations should be considered [14, 254–256]:
hepatitis B vaccination,
chickenpox vaccination,
herpes zoster vaccination,
seasonal influenza vaccination,
human papilloma virus vaccination (here the main target group is girls and possibly boys aged 11–12 years before sexual initiation),
pneumococcal and meningococcal vaccination.
Vaccination against COVID-19 (coronavirus disease 2019) should also be considered for all patients with IBD [14, 257, 258].
K. Psychological support
49. Psychological support should be made available to each patient with ulcerative colitis.
(Quality of evidence: very low; strength of recommendation: strong)
| Recommendation #49 – approval rating (Likert scale): | |||||
|---|---|---|---|---|---|
| 1 – complete disapproval | 2 – disapproval | 3 – partial disapproval | 4 – partial approval | 5 – approval | 6 – complete approval |
| 50% | 17% | 33% | |||
Ulcerative colitis has a significant negative impact on patients’ quality of life. Due to the prospect of living with an incurable disease, the fear of adverse drug reactions, surgical treatment and disability caused by the disease, as well as the bothersome symptoms, patients frequently experience depression and anxiety [14, 259]. Few studies have been carried out to date on the efficacy of various psychological interventions against these symptoms or on their impact on the course of IBD. So far, no data showing that any psychological intervention (such as behavioural and cognitive therapy) has an impact on UC remission rates have been published [14, 259–261]. Sparse evidence is available, however, to suggest that such interventions may improve the overall health of patients. For example, as shown in a randomised study by Wynne et al., acceptance and commitment therapy (ACT) significantly reduces the severity of anxiety or stress in IBD [14, 262]. Therefore, it appears that the possibility to obtain psychological support, as well as consideration being paid to the impact of UC on the patient’s emotional condition, should constitute an integral part of holistic care for IBD patients [14].










