Introduction
The immune system damages the liver cells in a condition known as autoimmune hepatitis (AIH), which results in inflammation, fibrosis, and liver failure [1]. Pediatric AIH is an autoimmune inflammation that necessitates long-term immunosuppression [2]. Relapses after stopping treatment frequently show that intrahepatic immunological mechanisms are not controlled by existing medications [3].
Autoimmune hepatitis is associated with female sex, high levels of immunoglobulin G (IgG) and aminotransferases, positivity for certain autoantibodies, and interface hepatitis on biopsy [4]. The failure of immune tolerance systems, environmental triggers, and genetic predisposition are some of the potential factors that may work together to cause a hepatic autoimmune attack [5].
Corticosteroids combined with azathioprine are the first-line treatment for AIH, and most patients react by going into remission [6]. Immunosuppressive treatment (IST) has a wide range of adverse effects and long-term morbidities, so the American Association for the Study of Liver Diseases practice guidelines advise patients with liver enzymes and IgG levels within the normal range for at least two years to consider IST withdrawal though relapse during or after IST discontinuation is frequent in AIH [7].
As a result, there is an unmet need for noninvasive blood-based biomarkers that could identify patients who are at a high risk of relapse or act as early indicators of recurrence [8]. Biomarkers with high clinical value are those that can predict the course of the disease clinically, identify individuals at risk for recurrence, and enhance medication dosing above and beyond existing practice [7].
Additionally, ferritin was researched as a potential biomarker for predicting therapy efficacy in AIH patients. A relationship between iron parameters and the result following a liver transplant and the responsiveness to AIH treatment is mentioned by Taubert et al. [9]. The development of operational tolerance following liver transplantation was similarly correlated with measures of intrahepatic iron homeostasis, according to Bohne et al. [10].
Hepcidin and ferritin were found to be differentially enhanced in individuals with operational tolerance after liver transplants, further demonstrating the relevance of iron homeostasis to inflammation and immunological tolerance [10].
Adults with severe vitamin D deficiency are more likely to have treatment failure, develop cirrhosis, or require liver transplantation [11]. According to Tao et al. [12], patients with AIH, particularly those with low vitamin D levels, are more susceptible to increased inflammatory and stress responses, low levels of T cell subsets, and a decline in immunity.
This study aimed to explore whether serum vitamin D and ferritin levels before starting AIH treatment have a role in disease prognosis regarding therapeutic response.
Material and methods
A prospective study was conducted on 100 children with AIH who attended the Pediatric Hepatology Department, National Liver Institute, Menoufia University during April 2020 to May 2021 and followed for a further 2 years. The National Liver Institute’s ethical committee approved the study proposal (Registration number: 00492/2023).
Any patient (< 18 years old) who met the simplified criteria for AIH diagnosis [13] was qualified for inclusion. Patients receiving vitamin D therapy before the study and those with chronic liver diseases other than AIH, such as hepatitis B and C, were excluded.
The patients received either corticosteroids alone (high dose steroids at 2 mg/kg/day tapering to reach a maintenance dose of 5 mg/day) or combined corticosteroids at 1 mg/kg/day plus azathioprine at 0.5 mg/kg/day). A complete response includes normalization of serum transaminases below the upper limit of normal (ULN) within 6 months after initiation of treatment. An incomplete response means a lack of serum transaminases normalization below the upper limit of normal within 6 months after initiation of treatment. A non-response was considered to mean less than a 50% drop in serum transaminases within 4 weeks of beginning therapy [14].
Remission was defined as a lack of symptoms, normal or near normal levels of liver blood tests, and improvement in the appearance of liver tissue (based on a biopsy). The initial period of remission generally occurs 12 or more months after treatment begins. Most people achieve remission by 18 months to three years of treatment [15].
Patient data and laboratory investigations
Data on patient’s age, sex, and complaint were collected. Laboratory investigations included serum bilirubin, aspartate transaminase, alanine transaminase using the Cobas 6000 analyzer (c501 module, Roche Diagnostics) and HCV antibody, HBV-sAg and HBV core IgG using the Cobas 6000 analyzer (e601 module, Roche diagnostic). Antinuclear antibody (ANA), anti-smooth muscle antibody (ASMA) and anti-liver kidney microsomal antibody type 1 (anti-LKM-1) were screened using Diasorin Inc CT3 immunoscreen Fluoro-kits (catalogue number 1740/1801) and interpretation was done with the Olympus fluorescence microscope BH2-RFL-T3 (Olympus).
The serum 25-hydroxy vitamin D (25-OH D) level was determined for all studied patients using Architect i1000SR (Abbott Diagnostics) while the ferritin serum level was determined by a Cobas 6000 analyzer (e601 module, Roche Diagnostics).
Liver biopsy
All liver biopsies from patients received prior to treatment were stained with Masson’s trichrome and hematoxylin and eosin (H&E). The Ishak score was used to grade the degree of inflammation and define the stage of liver fibrosis.
Liver biopsies were done at the end of treatment of AIH patients to confirm a response to treatment in only 25 patients who were compliant before treatment withdrawal.
Study outcomes
The serum ferritin and 25-OH D levels in children with AIH, as well as the correlations between these levels and the clinical, liver biopsy, liver biomarker, and biochemical characteristics of AIH, were determined.
The relationship between serum ferritin and 25-OH D levels and immunosuppressive medication response, as well as the reliability of serum ferritin and 25-OH D in predicting prognosis in children with AIH, was evaluated.
Statistical analysis
Both SPSS V.25 (IBM Corporation, 1 Orchard Rd, Armonk, NY 10504, USA) and Microsoft Excel 2019 (Microsoft Corporation, One Microsoft Way Redmond, WA 98052-6399, USA) were used to tabulate and statistically analyze the findings. The optimum cutoff value levels of serum ferritin and 25-OH D in the prognosis of children with AIH were found utilizing the ROC (receiver operating characteristic) curve employing sensitivity, specificity, positive predictive value, and negative predictive value. The 5% level was used to determine the significance of the obtained data.
Results
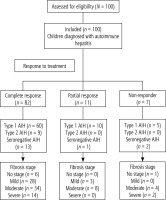
A flowchart of the study population is shown in Figure 1. All cases were divided according to stage of fibrosis and activity grading as described in Table S1. There was no significant relation between response to treatment and age, sex, AIH type or treatment line among the studied children (p > 0.05) (Table S2). There was a significant relation between response to treatment and fibrosis stage and grade of activity. Most of the complete response, partial response and non-response cases had moderate fibrosis (41.5%, 72.7%, 57.1%, respectively) (p = 0.047). Also, most of the complete response and partial response cases had mild activity (59.5%, 54.5%, respectively) and non-response cases had moderate activity (42.9%) (p = 0.042) (Table S3).
There were no significant differences among response groups regarding ANA and anti-LKM-1 antibody. It was found that 66 (80.49%) of complete response cases had positive ASMA, 10 (90.91%) partial response cases had positive ASMA and 3 (42.86%) non-response cases had positive ASMA (p = 0.038) (Table S4).
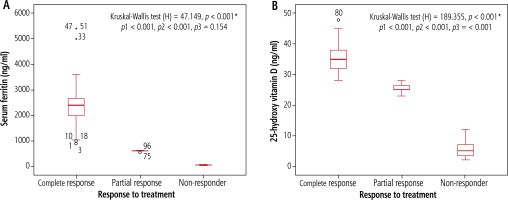
Serum ferritin and 25 hydroxy vitamin D levels were significantly higher among complete response (1000-3100 ng/ml, 29-48 ng/ml) than partial response (550-600 ng/ml, 23-28 ng/ml) and non-response (29.28-92.14, 2.16-8.72, respectively) cases (p < 0.001) (Fig. 2).
Fig. 2
Serum ferritin level (A), and 25-hydroxy vitamin D level (B) in relation to response of treatment among children with AIH. p1 – complete response vs. partial response, p2 – complete response vs. non-responder, p3 – partial response vs. non-responder
There were significant differences among AIH types regarding fibrosis stage and activity. Most of type 1 and 2 AIH cases had moderate fibrosis stage (50.7%, 66.7%) respectively (p = 0.026) also, cases with seronegative AIH had mild fibrosis stage (50%) (p = 0.026) (Table S5).
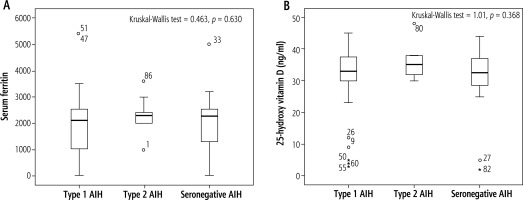
In this study, there were no significant differences among AIH types regarding serum ferritin and vitamin D (p > 0.05) (Fig. 3).
Serum ferritin level was significantly higher among severe fibrosis stage patients (2540) than mild and moderate (p = 0.015). Also, regarding activity grade, serum ferritin was significantly higher among severe activity grade (2830) than other activity grades (p = 0.004) (Table 1).
Table 1
Serum ferritin levels in children with autoimmune hepatitis (AIH) (N = 100) in relation to other studied clinical and laboratory variables
[i] There was no significant relation between vitamin D and sex, treatment, fibrosis stage, activity grade, confluent necrosis, ANA, ASMA or anti-LKM-1 (p > 0.05) (Table 2).
There was no significant relation between vitamin D and sex, treatment, fibrosis stage, activity grade, confluent necrosis, ANA, ASMA or anti-LKM-1 (p > 0.05) (Table 2).
Table 2
25-hydroxy vitamin D level in children with autoimmune hepatitis (AIH) (N = 100) in relation to other studied clinical and laboratory variables
[i] ROC curve analysis showed that the cutoff point of serum ferritin for prediction of prognosis in children with autoimmune hepatitis was 2325 ng/ml, with sensitivity of 96.3%, specificity of 71.0% at AUC of0.782, while the cutoff point of vitamin D for prediction of prognosis in children with autoimmune hepatitis was 33.5 ng/ml with sensitivity of 98.6%, specificity of 69.0% at AUC of0.720 (Table 3, Fig. 4).
ROC curve analysis showed that the cutoff point of serum ferritin for prediction of prognosis in children with autoimmune hepatitis was 2325 ng/ml, with sensitivity of 96.3%, specificity of 71.0% at AUC of 0.782, while the cutoff point of vitamin D for prediction of prognosis in children with autoimmune hepatitis was 33.5 ng/ml with sensitivity of 98.6%, specificity of 69.0% at AUC of 0.720 (Table 3, Fig. 4).
Fig. 4
ROC analysis of serum ferritin and vitamin D for prediction of prognosis in autoimmune hepatitis (AIH)
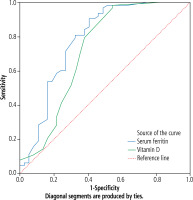
Table 3
ROC curve analysis of serum ferritin and 25-hydroxy vitamin D levels in prediction of prognosis in children with autoimmune hepatitis (AIH)
| Test result Variable(s) | AUC | Std. error | P value | Asymptotic 95% CI | ||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Cutoff value | Lower bound | Upper bound | ||||
| Serum ferritin (ng/ml) | 0.782 | 0.053 | < 0.001* | 96.3% | 71.0% | 2325 | 0.678 | 0.886 |
| 25-hydroxy vitamin D (ng/ml) | 0.720 | 0.060 | < 0.0001* | 98.6% | 69.0% | 33.5 | 0.603 | 0.837 |
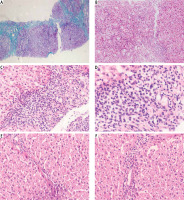
Pathological findings in pre- and post-treated autoimmune hepatitis (AIH) patients were showed in Figure 5.
Fig. 5
Pathological findings in pre- and post-treated autoimmune hepatitis (AIH) patients. A) A case of autoimmune hepatitis pre-treatment showing moderate fibrosis with porto-portal bridging (Masson’s trichrome 100×). B) A case of autoimmune hepatitis pre-treatment showing moderate portal tract inflammation (H&E 100×). C) A case of autoimmune hepatitis pre-treatment showing moderate interface (H&E 200×). D) A case of autoimmune hepatitis pre-treatment showing inflammatory infiltrate rich in plasma cells (H & E 400×). E) A case of autoimmune hepatitis post-treatment showing minimal fibrosis (H&E 200×). F) A case of autoimmune hepatitis post-treatment showing minimal inflammatory infiltrate (H&E 200×)
Discussion
Peri-portal hepatitis, hypergammaglobulinemia, and autoantibodies are signs of AIH [16]. For calcium and bone homeostasis, vitamin D, whose active form is 1,25-dihydroxy vitamin D3, is necessary. A lack of vitamin D has been linked to a few illnesses, including cardiovascular, autoimmune diseases, infectious diseases, and cancer [17]. Vitamin D insufficiency is frequently seen in patients with chronic liver disease, and this finding has been linked to severe fibrosis. In non-cholestatic liver disease, vitamin D insufficiency is typical and is correlated with disease severity [18]. Compared to the control group, vitamin D levels are low in AIH patients [19].
The liver serves as the primary organ for the storage of iron, making it one of its primary tasks. By synthesizing key proteins for iron metabolism, such as transferrin and the peptide hepcidin, the liver regulates the flow of iron into and around the body [20]. Chen et al. [21] found that higher serum ferritin levels were independently associated with advanced liver fibrosis in patients with treatment-naive autoimmune hepatitis.
Serum ferritin and 25-hydroxyvitamin D are higher in this study’s complete response group compared to partial response and non-response groups. However, there was no connection between the treatment’s effectiveness and the AIH score, fibrosis stage, or activity grade. It has been investigated how the vitamin D receptor and vitamin D resistance affect inflammation and autoimmune disease. According to Efe et al. [22], non-responders to IST had considerably lower baseline serum 25(OH)D levels than responders, and these non-responders also showed liver fibrosis and interface hepatitis. A recent large study by Ebadi et al. [11] confirmed these findings and found that patients with AIH and severe vitamin D deficiency (< 25 nmol/l) were more likely to have treatment nonresponse and liver-related mortality, making it a potential prognosticator at presentation.
Additionally, ferritin has been researched as a potential biomarker for predicting therapy efficacy in AIH patients. According to Taubert et al. [9], full biochemical remission was related to baseline ferritin levels that were higher than 2.09 ULN and immunoglobulin levels that were lower than 1.89 ULN. Hepcidin and ferritin were found to be differentially high in individuals with operational tolerance in patients with liver transplants, further demonstrating the relevance of iron homeostasis to inflammation and immunological tolerance [11]. It is important to note that both these markers are advantageous as they are readily available in clinical practice.
The results of the current study revealed that 50% of seronegative AIH cases had a mild fibrosis stage, whereas most of the type 1 and type 2 AIH cases had moderate fibrosis stage. Our study was comparable to Behairy et al.’s [23] study, which found that 70% of AIH cases had mild to moderate activity and 95% had moderate to severe fibrosis, and Pokorska-Śpiewak et al.’s [24] study, which found that most cases had minimal to mild fibrosis. However, Dhole et al. [25] found that 23% of patients had advanced fibrosis.
Interestingly, a high incidence of cirrhosis at diagnosis in children with AIH has been reported in several cases [26]. Also, of those who had not proceeded to cirrhosis, Ngu et al. [27] discovered that the majority had already experienced significant fibrosis. The fact that these young individuals were less likely to completely normalize their alanine transaminase (ALT) at six months or were more resistant to treatment as compared to patients who developed AIH in adulthood is even more concerning. These findings imply that the aggressive behavior of children and adolescents with AIH may necessitate a more aggressive management approach.
In our study, those with a mild fibrosis stage had considerably higher blood ferritin levels than those with severe and moderate stages, while the level of fibrosis had no significant relationship with vitamin D. In a similar vein, Chen et al. [21] discovered that in patients with treatment-naive autoimmune hepatitis, higher serum ferritin levels were independently linked with progressive liver fibrosis. AIH is a chronic, inflammatory liver disease that is immune-mediated and progresses over time. Liver fibrosis is a frequent complication of the development of AIH, and Mieli-Vergani and Vergani [28] reported that many patients have advanced liver fibrosis upon presentation. Additionally, Taubert et al. [9] found that serum ferritin levels and serum iron concentrations were elevated in 65% and 58% of individuals with untreated AIH, respectively. According to Shan et al. [29], serum ferritin levels matched serum aminotransferase levels in patients with chronic hepatitis C and AIH. Our research was comparable to that of Ebadi et al. [11], who found that 20% of white Canadian patients with AIH had severe vitamin D deficiency at the time of diagnosis, and that this condition was linked to the development of cirrhosis, a higher risk of dying from liver-related causes, and the requirement for liver transplantation. Indeed, the presence of complete or incomplete cirrhosis is the most frequent finding at diagnosis, occurring in 59% to 100% of pediatric patients [30] with extensive fibrosis, distortion of the hepatic lobule, and the appearance of regenerative nodules.
Limitation of the study
Larger sample sizes in subsequent research are required to support our findings.
Conclusions
This study showed a relation between serum vitamin D status before starting the specific treatment, the severity of AIH and response to therapy and suggested a relation of vitamin D status with response to therapy. This opens a new area of research on the potential use of vitamin D in children with AIH. Also, hyperferritinemia at diagnosis can predict the treatment response upon standard therapy. Additionally, the prognosis of AIH in children can be predicted at the level of serum ferritin of 2325 ng/ml (with sensitivity of 96.3% and specificity of 71.0%) and at level of serum vitamin D of 33.5 ng/ml (with sensitivity of 98.6% and specificity of 69.0%).