Introduction
Mutations in cancer cells generate new cancer cell-specific antigens (neoantigens), eliciting a host immune response against cancer cells [1]. In a favourable scenario, activated innate and adaptive immune cells may effectively eliminate neoplastic cells. However, tumours are capable of immune escape facilitating cancer development. The biomarker of the persistent immune response against cancer is the presence of tumour-infiltrating lymphocytes (TILs) inside the tumour (intratumoural TILs) and on its borders (stromal TILs). According to the recommendations of the International TILs Working Group TILs assessment should include the percentage of lymphocytes in the stroma within the invasive tumour borders, excluding areas of crush artifacts, necrosis, reactive stromal hyalinisation, and the site after a previous biopsy. Tumour-infiltrating lymphocytes should be evaluated as an averaged continuous parameter, without focusing on hotspots [2]. The abundance of TILs reflects the tumour mutational burden (TMB), which in breast cancer (BC) is average compared to other cancers, with only 5% of BC considered TMB high [3, 4]. The main component of TILs are T cells, B cells, monocytes, and NK cells, which usually account for about 75%, < 20%, < 10%, and < 5%, respectively [5]. The cellular composition and prevalence of TILs vary in different types of BCs. Some subpopulations promote whereas others suppress tumour progression. Inflammatory infiltration already occurs in benign ductal hyperplasia and ductal carcinoma in situ (DCIS) [6]. Interestingly, in DCIS dense TILs are associated with negative oestrogen receptor (ER) status and shorter recurrence-free survival (RFS) [7, 8]. Number of TILs, cellular composition of the inflammatory infiltrate, and expression of immune-related genes have predictive and prognostic significance in patients with HER2– amplified/overexpressing (HER2+ BC) and triple-negative breast cancer (TNBC). In the group of 294 BC patients, higher number of TILs was linked to ER– and HER2+ phenotype, larger tumour size, higher grade, higher Ki-67, and metastases to lymph nodes [9]. In the neoadjuvant setting high TILs in biopsy specimen predict favourable response to chemotherapy, and the presence of TILs in residual disease correlates with better outcomes in TNBC [10]. Similar trends were observed in HER2+ BC [11]. Much less is known about the role of TILs in hormone-dependent BC, especially single hormone receptor-positive BC (i.e. ER+/PgR– and ER–/PgR+). In a large study on ER+/HER2+ and ER+/HER2– tumours, high TILs were associated with unfavourable clinicopathological features but in patients treated with adjuvant chemotherapy correlated with longer distant disease-free survival (DFS) [12]. Interestingly, in the metasta- tic lesions, TILs are usually less abundant [13]. In a group of 94 patients with TILs assessed in BC metastases, lower abundance of TILs was found in younger patients, while higher TILs were correlated with prolonged overall survival (OS). Moreover, the composition of TILs differed depending on the location of the metastasis [14].
In BC the abundance of TILs correlates with immune-related gene expression profiles [15]. Interestingly, specific immunological profiles of BC microenvironment correlate with steroid hormone receptor status. Among luminal BCs, 3 subgroups were distinguished based on the expression of immune-related genes, one of which was characterised by higher amount of TILs and lower expression of ESR1/ESR2, supporting the relationship between weaker hormone receptor expression and enhanced immune reaction in BC [16].
In the current study, we aimed to investigate the role of TILs in single hormone receptor-positive BCs. We analysed associations with clinicopathological features, gene expression profiles, and long-term outcomes.
Material and methods
Study group
Tumours from 212 female patients diagnosed with single hormone receptor-positive BC were collected. Basic clinicopathological data (ER, PgR and HER2 status, Ki-67 proliferation index, grade, histological type, pathological tumour-node-metastasis [pTNM], use of neoadjuvant systemic treatment, age at diagnosis, date of diagnosis, date of relapse, and date of death) were retrieved from local databases. All ER–/PgR+ BC cases incorporated in the study had their phenotype adequately confirmed as described previously [17]. Subsequently, available microscopic slides were collected and subjected to analysis. Six patients were excluded from the subsequent analyses due to lack of slides for the assessment of TILs, leaving 206 patients in the study.
Evaluation of tumour-infiltrating lymphocytes
Assessment of stromal TILs was performed according to the International TILs Working Group guidelines by 2 pathologists (MK and RP) [2]. In the case of discrepant opinion, the final score was established after a discussion between pathologists. In general, one selected representative haematoxylin and eosin section from each case was analysed under 200–400× magnification. The percentage of the stromal TILs was assessed only within the invasive tumour borders. Tumour-infiltrating lymphocytes were scored as continuous variables and later subcategorised as low (0–5% of stromal TILs), and high (> 5% of stromal TILs).
RNA extraction
Total RNA was extracted from 52 samples prepared as formalin-fixed paraffin-embedded primary tumour tissue cores (4 cores of 0.6 mm diameter per patient, sampled from representative tumour areas) using the RNeasy Mini Kit (Qiagen, Germany) according to the manufacturer’s protocol. RNA integrity was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, US) with an Agilent RNA 6000 Pico Kit (Agilent Technologies).
nCounter gene expression assay
nCounter gene expression analysis technology was employed to decipher transcriptomic differences between single hormone receptor-positive BCs according to TILs abundance. Extracted RNA (4 µl) was pre-amplified using nCounter Low RNA Input Kit (NanoString Technologies, USA) with a dedicated Primer Pool covering sequences of 758 genes included in nCounter Breast Cancer 360 Panel (NanoString Technologies, USA). Pre-amplified samples were analysed using NanoString nCounter Analysis System (NanoString Technologies, USA) according to the manufacturer’s procedures for hybridisation, detection, and scanning, with BC 360 Panel Standard included in each run.
Background correction and normalisation were performed using nSolver 4.0 software (NanoString Technologies, USA). In brief, the background level was estimated by thresholding over the mean plus 2 standard deviations of negative control counts. Subsequently, the data were normalised according to the global mean of the counts of positive controls and the 3 most stably expressed housekeeping genes – OAZ1, TFRC, and PUM1. Breast Cancer 360 Panel Standard was used for batch calibration. The negative and positive control probes were included in the assay.
Following normalisation, low-expression genes (log2 me-- dian count in all samples < 6) were excluded, leaving 533 target genes for analysis. The collected RNA expression results were then analysed according to the TILs score of the corresponding primary tumour. Differences in gene expression between the TILs groups were presented as log2 fold change (log2FC). Genes with median-based log2FC ≥ 1 were considered as up-regulated and genes with log2FC ≤ –1 as down-regulated. Selected genes were associated with GO and Reactome terms using the STRING database [18].
Statistics
Statistical analyses were performed with the use of Statistica 13 (RRID:SCR_014213, Tibco, CA, USA) licensed to the Medical University of Gdańsk, and R statistical environment [19]. Categorical variables were compared by Fisher’s exact test or χ2 test with Yates correction. Continuous variables were analysed utilising the Wilcoxon test or Kruskal-Wallis test when applicable. Overall survival was defined as the time from the diagnosis to the date of death from any cause. Disease-free survival was defined as the time from the diagnosis to the date of relapse (either local or distant) or death and was analysed only in M0 cases (199 cases). Event-free survival (EFS) was calculated from the date of diagnosis to the earliest date of disease progression, relapse, or death from any cause. Kaplan-Meier curves were plotted to calculate the survival rates. The log rank test was used to compare survival between groups. Hazard ratios (HRs) were estimated using Cox regression analysis. A p-value < 0.05 was considered significant; in cases of multiple comparisons, p-values were adjusted with Benjamini-Hochberg correction. Boxplots and scatterplots were produced using the ‘ggplot2’ and ‘ggstatsplot’ packages [20, 21]. Kaplan-Meier curves were plotted using the ‘survminer’ and ‘ggsci’ packages [22, 23].
Results
In the study group, 130 (63.1%) patients were classified as ER+/PgR– and 76 (36.9%) as ER–/PgR+ (Table 1). The frequency of HER2 overexpression/amplification did not differ between groups (p = 0.718, χ2). The mean age of the patients was 61 years (range 28–91, interquartile range, IQR 53–69). Patients in ER+/PgR– were significantly older (p = 0.0218, Wilcoxon test), and their tumours had lower Ki-67 index (p < 0.001, Wilcoxon test). Tumours with ER–/PgR+ phenotype more commonly displayed grade 3 histology when compared to ER+/PgR– cancers that were predominantly grade 2 (p < 0.001, χ2). Neoadjuvant treatment was administered to 34 (44.7%) patients in the ER–/PgR+ group and 9 patients (7%) in the ER+/PgR– group (p < 0.001, χ2). ER–/PgR+ tumours were significantly larger (p < 0.001, Wilcoxon test) and had higher pT descriptors in TNM (p = 0.044, χ2). Moreover, ER–/PgR+ tumours were more commonly associated with the presence of distant metastases (p = 0.048, χ2). However, there was no significant difference in the frequency of nodal metastases (p = 0.187, χ2). Median follow-up was 38.5 months (IQR 21.5–76 months). Twenty-four (18.5%) ER+/PgR– patients died and 22 (16.9%) had a relapse/progression during follow-up, compared to 12 (15.8%) and 11 (14.5%) ER–/PgR+ patients, respectively. There were no significant differences in OS, DFS, and EFS between ER+/PgR– and ER–/PgR+ patients. When subcategorised according to HER2 status, ER–/PgR+/HER2+ patients had the best OS, followed by ER+/PgR–/HER2– patients, while ER+/PgR–/HER2+ and ER–/PgR+/HER2– patients had poorer OS (p = 0.006, log rank).
Table 1
Main clinicopathological features of the study groups
Tumour-infiltrating lymphocytes score
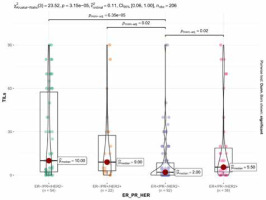
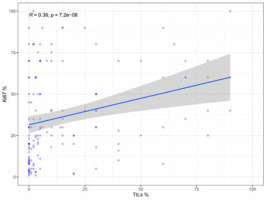
Fifty-three (25.7%) cases were scored as TILs negative. The remaining cases showed at least 1% of stromal TILs (median 4%, mean 16.3%, IQR 0–20%). ER–/PgR+ tumours displayed significantly denser TILs than ER+/PgR– cases (p < 0.001, Wilcoxon test), irrespectively of HER2 status (Fig. 1). HER2 overexpression was associated with higher TILs but only in the ER+/PgR– subgroup (as a dichotomised variable – p = 0.006, χ2 and as a continuous variable – p = 0.003, Wilcoxon test). The abundance of TILs positively correlated with higher grade (p < 0.001, Kruskal-Wallis), higher Ki-67 (p < 0.001, R = 0.36, Spearman) (Fig. 2), and younger age (p = 0.008, R = –0.19, Spearman) but not with tumour size (p = 0.54, R = –0.04, Spearman), status of regional lymph nodes (p = 0.983, Wilcoxon test), and distant metastases at the time of diagnosis (p = 0.841, Wilcoxon test).
Association of tumour-infiltrating lymphocytes status with long-term outcomes
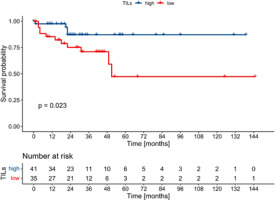
Tumour-infiltrating lymphocytes percentage as a continuous variable was associated with better EFS with borderline statistical significance (HR 0.98, 95% CI: 0.96–0.99, p = 0.043, Cox), and it had no impact on DFS (HR 0.98, 95% CI: 0.96–1.0, p = 0.083, Cox) and OS (HR 0.98, 0.97–1.0, p = 0.102, Cox). When dichotomised, high-TILs (i.e. > 5%) correlated with better EFS in the whole cohort, as well as in the ER+/PgR– and ER–/PgR+ subgroups (Figs. 3–5). The 5-year EFS rate in the whole cohort was 87% in the high TILs group, and 67% in the low TILs group (p = 0.001, log rank). Significant differences in OS were noted in the whole cohort and in the ER–/PgR+ subgroup, whereas significant differences in DFS were observed in the whole cohort and in the ER+/PgR– subgroup (Suppl. Figs. 1–6).
Fig. 3
Kaplan-Meier curves for event-free survival in the whole co-hort according to tumour-infiltrating lymphocytes status
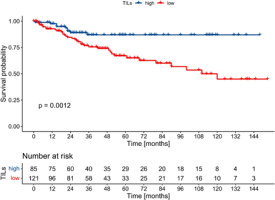
Fig. 4
Kaplan-Meier curves for event-free survival in ER+/PgR– patients according to tumour-infiltrating lymphocytes status
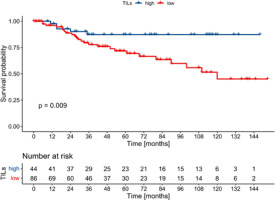
Fig. 5
Kaplan-Meier curves for event-free survival in ER–/PgR+ patients according to tumour-infiltrating lymphocytes status
Multivariate analysis was restricted to EFS due to insufficient numbers of DFS and OS events. In the whole cohort nodal status, the presence of distant metastases, TILs, and Ki-67 index were associated with EFS in multivariate analysis (Table 2). In the analysis restricted to ER–/PgR+ patients, only TILs status and distant metastases were associated with EFS (Table 3), whereas in ER+/PgR– patients, T-descriptor, nodal status, HER2, and TILs correlated with EFS (Table 4).
Table 2
Multivariate Cox regression analysis for event-free survival in the whole cohort
Table 3
Multivariate Cox regression analysis for event-free survival in ER–/PgR+ cohort
Table 4
Multivariate Cox regression analysis for event-free survival in ER+/PgR– cohort
Association of tumour-infiltrating lymphocyte status with gene expression profile
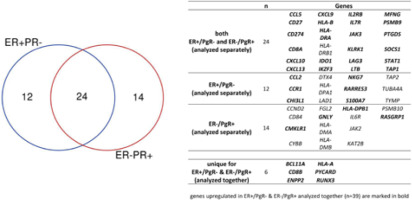
The analysis covered 758 genes associated with BC biology, microenvironment, and immune response. When ER+/PgR– and ER–/PgR+ cases were analysed together, 50 DEGs were identified, including 39 up- and 11 down-regulated genes in the high TILs group (Table 5). The most upregulated genes included CXCL13 (C-X-C Motif Chemokine Ligand 13), BCL11A (BCL11 Transcription Factor A), CD27 (CD27 Molecule), GABRP (γ-aminobutyric acid type A receptor subunit Pi), GNLY (granulysin), and KLRK1 (killer cell lectin-like receptor K1). The genes with higher expression in high TILs tumours were associated with immune response, including NK chemotaxis regulation, antigen presentation, chemokine receptor binding, or interleukin and interferon signalling (Suppl. Table 1). Importantly, the upregulated expression of immune-related genes was consistent also across single hormone receptor-positive groups analysed separately (ER+/PgR–, ER–/PgR+) (Fig. 6). On the other hand, TILs-high tumours showed reduced expression of genes involved in tight-junction formation (CLDN3, OCLN), most probably resulting from lower abundance of epithelial cells in tissue cores sampled from high-TILs tumours.
Table 5
Differently expressed genes identified for tumour-infiltrating lymphocytes high (n = 25) vs. tumour-infiltrating lymphocytes low (n = 27) comparison in single hormone-receptor positive breast cancers (ER+/PgR– and ER–/PgR+ cases analysed together)
Fig. 6
Venn diagram presenting the overlap of upregulated genes identified for tumour-infiltrating lymphocytes high vs. tumourinfiltrating lymphocytes low comparison in single receptor-positive breast cancers analysed together and separately
The only gene that had significantly different (higher) expression among TILs-high ER–/PgR+ (n = 14), comparing to TILs-high ER+/PgR– tumours (n = 11) was SOX10 (log2FC = 7.75, p = 0.0201, Wilcoxon test).
Discussion
Single hormone receptor-positive BC accounts for about 15–20% of BCs, and the vast majority display the ER+/PgR– phenotype, whereas ER–/PgR+ tumours are very rare [24]. The latter is a controversial group, and many authors disregard ER–/PgR+ cancers as technical artifacts. Moreover, given the infrequency of ER–/PgR+ tumours, there is a significant scarcity of studies dedicated to them, and we were able to include a relatively substantial number of such cases in our study. Nevertheless, multiple studies have confirmed their existence and demonstrated similarities to TNBC [25]. Therefore, the assessment of predictive and prognostic factors in this subpopulation is of great clinical importance. ER–/PgR+ BC are more common in younger women, they are more often poorly differentiated, larger, more frequently have high proliferation index, positive lymph node status, distant metastases, and high glucose metabolism, and carry worse prognosis compared to ER+/PgR+ tumours [26, 27]. Similarly, in the current study, ER+/PgR– BCs occurred in older patients and had lower Ki-67 index, while ER–/PgR+ BCs were larger, had higher grade, were more likely to have high TILs, and were more often associated with distant metastases. In one study survival in both groups of single hormone receptor-positive BCs was similar, although in ER–/PgR+ cancers higher expression of the PgR was associated with longer RFS and DFS [28]. On the contrary, in our cohort of ER–/PgR+/HER2– BCs, a negative prognostic significance of higher PgR expression was demonstrated [17]. In ER+ BCs a lack of PgR expression is predictive for higher incidence of pCR after neoadjuvant chemotherapy and poorer response to anti-ER treatment [26].
ER-positive BCs are less immunogenic compared to hormone receptor-negative BCs; therefore, they are less likely to have high TILs [8, 29], which may explain the lower prevalence of intermediate/high TILs in ER+/PgR– cancers in our study. Data on the prognostic and predictive value of TILs in BC are inconsistent. Moreover, there are few data on the prognostic significance of TILs in single hormone receptor-positive BCs. Most of the available data relate to triple-negative and HER2+ cancers, in which higher TILs are associated with better response to neoadjuvant chemotherapy and improved prognosis; however, in luminal BC high TILs seem to be a negative prognostic factor [30–32], including worse OS [9]. This unexpected correlation may be explained by confounding effects of age, grade, and stage on the prognostic significance of TILs [32]. In the current study high TILs as a continuous parameter correlated positively with EFS in both groups of single hormone receptor-positive BCs. Alike in TNBC, we demonstrated that also in single hormone receptor-positive BCs, higher TILs are associated with higher tumour grade [7].
There are differences in gene expression among high and low TILs BCs. In the group of 200 HER2+ BCs, high TILs were associated with up-regulation of immune genes, down-regulation of stromal and ER genes, and better response to trastuzumab [33]. Similarly, in our group of single hormone receptor-positive BCs, we identified a relationship between high TILs and higher expression of immune-related genes, and lower expression of stromal genes. One of the immune-related genes which were up-regulated in our cohort is CXCL13, which plays an important role in the formation of organised structures composed of B, T lymphocytes, and dendritic cells – tertiary lymphoid structures (TLSs), which are involved in the immune response against cancer cells. CXCL13 has a prognostic significance in BC, especially in HER2+ BC, positively affecting DFS [5]. In addition, CXCL13/CXCR5 signalling is of significant prognostic importance in TNBC immunotherapy with atezolizumab [34].
Another interesting finding is the difference in SOX10 expression between TILs-high ER–/PgR+ and ER+/PgR– tumours. SOX10 is a useful immunohistochemical marker of both primary and metastatic TNBC even if standard breast lineage markers are reduced [35]. In a study on HER2+/ER– BC SOX10 expression was associated with features typically seen in TNBC, i.e. large acellular zone or prominent TILs [36]. On the other hand, HER2+/ER+ tumours are almost exclusively SOX10-negative [37]. Thus, higher expression of SOX10 mRNA in TILs-high ER–/PgR+ tumours may indicate their phenotypic resemblance to other ER– BCs, and future studies should elucidate the role of SOX10 as a potential biomarker of ER–/PgR+ BC.
The optimal cut-offs for TILs in various types of BC can vary depending on the specific subtype and the assessment method used. It is important to note that the field of TILs and their prognostic or predictive value in BC is still evolving, and there are no universally accepted cut-offs for all subtypes. It ranges from 5 to 75% in different research studies or clinical trials, and the cut-offs are generally higher in HER2+ BCs and TNBC compared to luminal BCs [12, 38–41]. In our study we did not predefine the cut-off for TILs assessment, and long-term outcomes analysis was based on the median TILs percentage identified in our cohort, and this is a potential limitation of our study.
Other major limitations are the study’s retrospective nature and the small size of the cohort. Moreover, while we assessed only chemo-naïve cases, in some instances, TILs were assessed in biopsy material or surgical specimens, depending on the availability of specimens, which might have impacted the results. Thus, our findings should be analysed with caution.
Conclusions
Our study confirms that single hormone receptor-positive BCs and both their subgroups (ER+/PgR– and ER–/PgR+) are biologically distinct groups of BCs in which the assessment of TILs carries prognostic significance. Additionally, in patients with single hormone receptor-positive BC the presence of TILs is associated with distinct gene expression profiles and better outcomes. Our findings suggest that the assessment of TILs should be considered as part of routine assessment of single hormone receptor-positive BC, although the role of TILs in clinical decision-making in these BC subtypes warrants further research. Future studies should validate these findings in larger cohorts and address the potential role of TILs as a predictive factor in these tumours.