Therapy with cardiac implantable electronic devices (CIEDs) carries a risk of various complications such as lead perforation, lead-related endocarditis (LRIE) or lead-dependent tricuspid dysfunction (LDTD). One of the main mechanisms of LDTD is abnormal leaflet coaptation caused by loop of the lead and intensive lead impingement of the leaflets [1].
Removing the lead that prevents coaptation of the leaflets creates a chance to restore the function of the tricuspid valve in cases where there are no significant degenerative changes of the leaflets or excessive dilatation of the tricuspid annulus [2].
A 71-year-old woman with a single chamber ventricular pacing system (VVI) implanted 10 years previously due to tachy-brady syndrome with Morgagni-Adams-Stokes syndrome in the course of atrial fibrillation was hospitalized several times recently due to symptoms of severe heart failure with only reduced left ventricular ejection fraction (LVEF 45%).
In the past she had two commissurotomies (in 1993 and 1996) due to severe mitral stenosis, then in 2017 she had cardiac surgery – mitral valve replacement (mechanical valve St. Jude Medical 27 mm).
Ten years ago she had an ischemic stroke with left-sided hemiparesis and 2 years ago she had a subarachnoid hemorrhage. Recently she was admitted to the cardiac surgery department with symptoms of heart failure in class III according to the New York Heart Association, dyspnea and peripheral edema.
Echocardiography (transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE)) showed significant tricuspid regurgitation (vena contracta (VC) 18 mm; regurgitant volume (R vol) 110 ml; effective regurgitant orifice area (EROA) 4.2 cm²; large right atrium 41 cm²) due to abnormal leaflet coaptation caused by loop of the lead in the tricuspid valve. Additionally, she had enlargement of the right ventricle and dilatation of the tricuspid annulus (Figures 1 A, B).
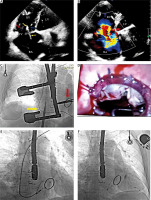
Figure 1
A – TTE, apical four-chamber view (4CH). The lead (yellow arrows) supports the septal leaflet (blue arrow), making coaptation with the anterior leaflet (red arrow) difficult. The tricuspid valve ring was dilated to 45 mm. B – TTE, 4CH, color Doppler. Massive tricuspid regurgitation. C – Fluoroscopy – yellow arrow – the lead; red arrow – artificial mitral ring. D – Intraoperative view: sewn-in ring, lead. E – Fluoroscopy – removal of the lead using a Cook catheter system. F – The new single lead implanted through the coronary sinus (CS) to bypass the tricuspid ostium
She was scheduled for hybrid treatment – mini-invasive tricuspid valvuloplasty (right lateral minithoracotomy) and then transvenous lead extraction (TLE).
The chest was opened through a right lateral minithoracotomy in the fourth intercostal space. Adhesions were observed in the right pleura after previous surgery (Figure 1 C). After heparin administration, cannulation of the femoral vein (under the scope) and the right femoral artery was performed. The patient was connected to a cardio-pulmonary bypass pump. A cannula was inserted into the superior vena cava and the venous drainage was divided into the superior and inferior vena cava. The right atrium was incised on the beating heart, revealing the tricuspid valve – a very dilated annulus, a huge right atrium and lead pressing on the septal leaflet and a connection between the lead and the leaflet with strong connecting scar tissue. The lead was released from the leaflet and chordae tendineae in the right ventricle. Tricuspid valvuloplasty was performed with implantation of an artificial C-E MC3 34 mm ring; CorKnot automatic sutures were used (Figure 1 D). The effectiveness of plastic surgery was checked using a water test. The right atrium was closed with a continuous suture. After the operation, the drapes and surgical tools were changed and the rooms were washed. The patient remained anesthetized. During the same anesthesia after deheparinization TLE was performed. We used different polypropylene Byrd dilator unpowered sheaths for lead dilatation on its course through the venous and atrial route (Figure 1 E). A new single left ventricular lead was implanted into the branch of the coronary sinus and a new pacemaker was connected (Figure 1 F).
In the following days, symptoms improved. A follow-up echocardiogram revealed severe tricuspid valve regurgitation. Typical heart failure treatment (spironolactone, bisoprolol, ACE inhibitor, flozin, diuretics) and acenocoumarol were included.
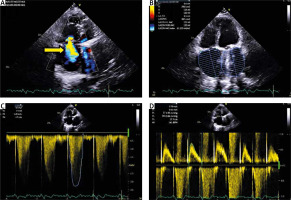
She was discharged home in good condition, with subjective improvement of symptoms NYHA I/II. Three months after the surgery, the patient underwent a follow-up echocardiogram. TTE showed a significant reduction in tricuspid valve regurgitation (VC 7 mm, R vol 73 ml; EROA 0.6 cm²; large right atrium 30 cm²), reduction of right ventricular diameter from 49 mm to 35 mm and improvement in the longitudinal strain of the right ventricle from –11.1% to –15.6% (Figure 2).
Figure 2
A – TTE, 4CH, color Doppler. Severe tricuspid regurgitation – yellow arrow. B – TTE, 4CH. Dimension of the right atrium 30 cm². C – TTE, continuous-wave Doppler (CW). Tricuspid regurgitation, TR Vmax 3.28 m/s. D – TTE, pulsed-wave Doppler (PW). Inflow through the tricuspid valve, tricuspid valve max 0.7 m/s
Patients with LDTD constitute a significant problem for electrocardiologists and cardiac surgeons [1, 3]. In practice, diagnoses are made with a considerable delay, because only in the last dozen or so years have echocardiographers begun to pay attention to the role of the right ventricular lead or atrial lead loop in impeding leaflet coaptation. Even with a correct diagnosis, patients often do not consent to the removal and replacement of the lead for a long time, most often giving consent when large doses of diuretics do not eliminate the swelling. The classic “initial” procedure is to remove the lead causing valve dysfunction and, if ventricular pacing is necessary, implant a new one that does not interfere with the tricuspid apparatus (into the CS branch for transvenous epicardial stimulation of the left ventricle). The procedure creates a chance to activate the supported flap and improve the function of the trigeminal apparatus if the degenerative changes are not too severe [4]. If the result of replacing the lead does not meet expectations, valvuloplasty should be considered. This case highlights the importance of close collaboration between the cardiologist and the cardiac surgeon during transvenous lead extraction [5].
In our patient’s case, the chance of improving valve function was negligible, and therefore we reversed the procedure: tricuspid valve plasty, TLE and left ventricular lead implantation. Right-sided thoracotomy excludes the possibility of more hemodynamically favorable conventional epicardial left ventricular pacing, and in our patient’s case, adhesions in the pericardium after two cardiac surgical interventions in the past eliminated the possibility of epicardial pacing even in the case of sternotomy.
For patients with serious, long-term LDTD, the optimal treatment option seems to be a hybrid procedure – performing tricuspid valve plasty and replacing the right ventricular lead with a left ventricular lead during one anesthesia administration.






