IgG4-related disease is a systemic, immune-mediated disease with multi-organ involvement. Aortic involvement is sometimes complicated by dilatation and/or inflammatory aneurysms. Due to the heterogeneity of its manifestations, it is necessary to integrate clinical, laboratory, instrumental and histopathological data for a definitive identification. The four main pathological signs of IgG4 disease are dense polyclonal lymphoplasmacytic infiltrates with high percentages of IgG4+ plasma cells, storiform fibrosis, obliterative phlebitis and tissue eosinophilia [1, 2].
A 66-year-old man was admitted to the emergency room for angina pectoris, followed by a syncopal episode. His medical history included smoking, hypertension, dyslipidemia on pharmacological treatment and previous surgery to remove a pituitary adenoma. The following coronarography revealed chronic occlusion of the right coronary artery and critical stenosis of the ostial left anterior descending (LAD), so myocardial revascularization surgery was suggested and the patient was transferred to our facility. Upon admission, the patient was afebrile, blood pressure (BP) was 110/65 mm Hg, heart rate (HR) 90 bpm, spO2 96% (FiO2 0.21). The cardiovascular examination was within the limits; peripheral pulses were present, valid and isosphygmic. Chest X-ray and electrocardiogram were within normal limits as well. Echocardiography showed a left ventricle of normal size and wall thickness, good global and segmental kinesis and a double-track image of the ascending aorta which was slightly ectatic. Therefore, we decided to further investigate the condition of the aorta with second and third level tests in order to choose the best surgical approach, the type of ducts to be harvested and the number of bypasses to perform. A computed tomography angiogram (CTA) of the thorax and abdomen was carried out, demonstrating an ectatic ascending aorta with the inner lumen measuring 3.84 cm (Figure 1 A). It also showed concentric wall thickening of the ascending aorta with adjacent fat stranding (Figure 1 B). Then, a cardiac MRN highlighted significant diffuse dilatation of the thoracic aorta, largely attributable to widespread wall thickening and morphological and tissue characterization elements overall attributable to extensive phlogistic involvement. Therefore, a 18F-FDG-PET examination was additionally performed; it was positive for vasculitis affecting the thoracic aorta and suggestive of metabolically active atherosclerotic degeneration affecting the infrarenal abdominal aorta. Infectious and immunological tests were performed for etiological definition of the aortic inflammatory process. Blood tests showed increased C-reactive protein (10 mg/l, with v.n. < 5 mg/l), a significant increase in a IgG4 immunoglobulin subclass equal to 122 mg/dl (v.n. < 86 mg/dl) and in p-ANCA with a titer of 1 : 160. No ulcerative lesions were found in the oral cavity or external genitalia compatible with the diagnosis of Behçet’s disease, nor pain or tenderness in the temporal artery region that could be attributed to Horton’s arteritis. Considering the inflammation of the aortic walls, the increased IgG4 values and the absence of diagnostic criteria for other specific vasculitis, a diagnosis of possible IgG4-related disease was made. Once the IgG4-related aortitis was diagnosed, an off-pump bypass with double mammary artery was opted for. Usually we do not perform subclavian artery Doppler echo as routine preoperative examination and we did not perform Doppler in this case either. Intraoperatively, upon exploration of the native coronary arteries, the right coronary artery appeared to be of an unsuitable size for revascularization, so only left internal mammary artery (LIMA) anastomosis on the LAD off pump was performed. Intraoperatively, the aorta demonstrated concentric wall thickening with dense white fibrosis and adhesions (Figure 2). No intramural hematoma was seen. During the operation, a segment of the mammary artery was used for histological study. There was no sign of perivascular fibrosis on the LIMA graft. Histological examination showed dense lymphoplasmacytic infiltrate, fibrosis and an increased number of IgG4 plasma cells. The postoperative hospital stay was approximately 10 days, without any complications. Taking into account the patient’s asymptomatic nature and the normalization of inflammation indices, it was decided not to start any cortisone or immunosuppressive therapy. Upon discharge, the patient was advised to have a rheumatological/immunological examination for a possible global investigation.
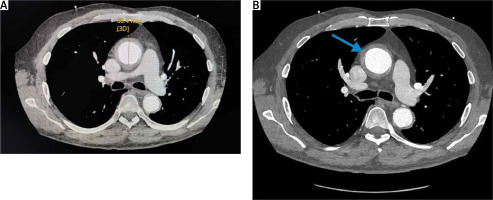
Figure 1
A – Axial post-contrast CTA thorax: ectatic ascending aorta with the inner lumen measuring 3.84 cm. B – Axial post-contrast CTA thorax demonstrates circumferential soft tissue thickening (blue arrow)
Our case describes a condition discovered by chance, of which the patient was unaware as he had never presented symptoms. Although the exact pathophysiology of IgG4 aortitis is still unclear, in 2018 Kasashima proposed an inflammatory pathway that activates T helper cells which, in turn, release various cytokines, plasma cells and fibrosis. T helper cells attract into the adventitia IgG4-positive B lymphocytes which subsequently differentiate into plasma cells. Infiltration of plasma cells into the adventitia leads to destruction of the elasticity of the aortic wall and periadventitial remodeling, which could lead to wall weakness and predispose to aneurysm and dissection [1, 3]. Aortic dissection associated with IgG4-RD is not a rare finding, but it may often be underdiagnosed, considering the limited available data. The significance of IgG4-RD in the pathogenesis of aortic dissection should be investigated in future studies [4]. Upon direct vision, as in our case, the aorta shows a bluish or whitish superficial color and is hard upon palpation. Histologically we find lymphoplasmocytes with storiform fibrosis, obliterative phlebitis, IgG4/IgG positive plasma cell ratio > 40% and IgG4 positive plasma cell count > 10/hpf (high power field). CTA shows soft tissue thickening of the aortic wall which can often be mistaken for intramural hematoma [3, 4]. The diagnostic criteria used for the diagnosis of IgG4 aortitis are: 1) hypertrophic lesion of the arterial wall on imaging findings, 2) increased serum IgG4, 3) histopathological findings. It is a definitive diagnosis if all three criteria are present [5]. The differential diagnosis is important and often complicated, especially in patients with non-specific signs. The differential diagnosis of circumferential aortic wall thickening includes intramural hematoma, non-infectious aortitis and infectious aortitis [1]. Treatment of IgG4 aortitis remains controversial due to the lack of randomized clinical trials. No international recommendations or guidelines on the treatment of this type of aortitis or on any surgical treatment have been reported in literature. Several studies have shown that medical therapy with steroids can improve aortitis both clinically and radiographically [6].
In our case, it was decided not to intervene either surgically or with steroid therapy for the treatment of aortitis but to surgically treat only the coronary problem. In clinical practice, the radiological finding of thickening of the aortic walls with post-contrast enhancement should raise the diagnostic suspicion of aortitis. The radiological suspicion must then be ascertained with second and third level tests such as PET and MRI associated with specific laboratory investigations and histological study, if possible.