Introduction
Gallbladder malignancy has high incidence rates in specific geographical regions of the world and is an understudied neoplasm [1]. Early diagnosis and definitive surgery offer the only hope in these patients as locally advanced and unresectable disease have an abysmal prognosis [2, 3]. The overall 5-year survival rate in gallbladder cancer (GBC) patients is 80% in patients with the in situ disease. It declines to only 8% in cases of lymph nodal involvement and 2% for patients with stage 4b disease [4]. These figures demonstrate the need for early diagnosis to prevent early spread. Three morphological forms of gallbladder cancer have been described. A mass replacing the gallbladder is the most common type. Around 20% to 30% of patients with GBC have gallbladder thickening [5, 6]. However, gallbladder wall thickening is highly non-specific, commonly encountered in imaging, and can be secondary to a wide range of local and systemic pathologies [7]. Unfortunately, there is a lack of literature on the wall thickening type of GBC. Awareness regarding the patterns of mural involvement and extramural spread may aid radiologists in the early and accurate differential diagnosis. This review elaborates on the various patterns of involvement and spread in the thickening type of GBC.
Patterns of wall thickening
Gallbladder wall thickness > 3 mm is considered pathological [8]. Gallbladder wall thickening can be focal or diffuse. Diffuse gallbladder wall thickening is usually a manifestation of benign and inflammatory disorders [9, 10]. On the other hand, focal wall thickening is due to an intrinsic gallbladder pathology in the majority of patients [8]. Gallbladder cancer can present as both focal and diffuse thickening, with the focal pattern being more common. The diffuse pattern of GBC can be symmetrical or asymmetrical. The presence of asymmetrical thickening with loss of integrity of the mucosa and loss of the layered appearance that can be easily identified on high resolution ultrasound (HRUS) helps us identify early GBCs and differentiate them from innocuous thickening [11-13]. This is depicted in Figure 1. In the case of the absence of the findings mentioned above on conventional modalities, the high SUV values on fluorine-18-fluorodeoxyglucose positron emission tomography (FDG PET) can be used to identify the diffuse type of GBC [14].
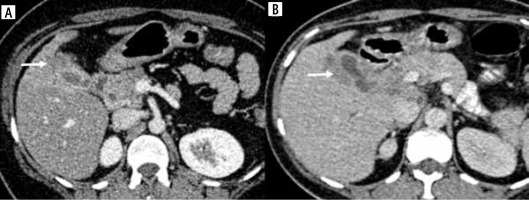
Fig. 1
Pattern of thickening in gallbladder cancer (GBC). A) Symmetrical circumferential thickening involving the GB which is infiltrating into the adjacent liver parenchyma (arrow). B) Asymmetrical mural thickening involving the GB with multiple calculi which is infiltrating the liver parenchyma (arrow). C) Asymmetrical mural thickening involving the fundus of the GB with maintained interface with adjacent liver surface (arrow). Ascites was also seen in this case (blue arrow)
Location of wall thickening
Focal gallbladder thickening can be seen in the fundus, body, or the neck of the gallbladder. Thickening in the neck region most often poses a diagnostic challenge in differentiation from other biliary tract malignancies such as cholangiocarcinoma [15]. Cross-sectional imaging with angiography and cholangiography facilitates the assessment of vascular and biliary involvement and helps differentiate these two entities.
It is also essential to differentiate wall thickening in the fundus region from normal variants such as a mucosal fold or a Phrygian cap, identified in 4% of cases [16]. HRUS evaluation in at least two different planes is mandatory to pick up focal gallbladder wall thickening. Multiphase computed tomography (CT) or magnetic resonance imaging (MRI) also performs well in differentiating between the two entities [17]. At times, focal thickening in the fundus region creates a diagnostic dilemma between GBC and the most common type of focal adenomyomatosis, which also involves the fundus of the gallbladder. Identification of cystic spaces suggestive of bile-filled Rokitansky-Aschoff sinuses is crucial for an imaging diagnosis of adenomyomatosis [18].
Focal GBC in the body is seen along the hepatic surface (at least a part of the tumor is in contact with the liver) or the peritoneal surface (without contact with the liver surface) [19]. This may influence the pattern of regional spread to the liver or the surrounding viscera/peritoneum. A few studies have shown that the tumor’s location on the hepatic side poses a poor prognosis due to ease of direct invasion into the liver as a result of absent serosa on this side [19-21]. Toge et al. reported a higher frequency of lymphatic vessels on the hepatic side, resulting in a higher incidence of lymph nodal metastasis in such cases [22]. The various locations of malignant gallbladder wall thickening are illustrated in Figure 2.
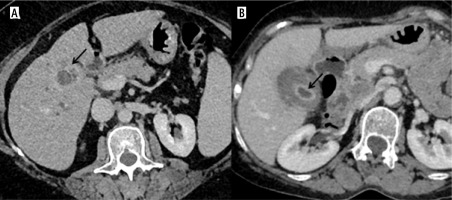
Fig. 2
Different locations of gallbladder cancer (GBC) thickening type of GBC. A) Homogeneous thickening at GB fundus showing ill-defined fat planes with liver (arrow). B) Asymmetrical mural thickening at the neck region of the GB which is infiltrating into the adjacent liver parenchyma (arrow). C) Axial upper abdomen section shows diffuse circumferential thickening of the entire GB wall (arrow)
Intramural characteristics
Focal smooth polypoidal wall thickening of < 1 cm is often benign and may contain internal echogenic foci [12, 23]. In contrast, polypoidal wall thickening > 1 cm, hypoechogenicity, and internal hypoechoic foci favor malignant wall thickening [24]. Focal segmental or annular thickening with intramural cystic spaces or echogenic foci with comet tail artifacts favor benign etiology and are characteristic for adenomyomatosis [12]. Preserved mural stratification of the gallbladder wall is seen in benign inflammatory disorders of the gallbladder [21]. The presence of mural calcification is considered premalignant, and such cases undergo prophylactic cholecystectomy. However, recent literature has raised doubts regarding this and has reported a significantly lower incidence than previously reported [25]. The risk of carcinoma is 6-7%, significantly lower than the previously reported incidence, as high as 61% [26, 27].
Patterns of diffusion restriction and enhancement
A few specific patterns of enhancement have been reported for GBC cases. Kim et al. reported five different patterns of layered mural enhancement in diffuse GBC thickening [28]. They suggested the two-layer pattern with strong enhancement of the thick inner layer (≥ 2.6 mm) and weak enhancement of the outer layer (≤ 3.4 mm) and the one-layer pattern with a heterogeneous and thick enhancing wall to be malignant patterns. Corwin et al. reported six different enhancement patterns in the focal type of thickening [29]. Type 3 (enhancement of the entire focal fundal thickening) and type 6 (heterogeneous enhancement of the focal fundal wall thickening without discrete cystic spaces) were significantly associated with malignant cases, with type 6 being more common than type 3. These enhancement patterns may be better depicted on iodine overlay maps in dual energy CT (DECT) [30, 31] (Fig. 3). Several studies have shown that the malignant cases show early contrast enhancement due to neovascularization on dynamic contrast-enhanced MRI [32]. Contrast enhanced US (CEUS) is also increasingly used to identify the malignant thickening, which shows early phase washout with persistent hypoenhancement in the late phase [33].
Fig. 3
Patterns of enhancement in wall-thickening type of gallbladder cancer (GBC). A) Heterogeneous enhancement (arrow). B) Bilayered appearance with thick enhancing inner layer (arrow)
In diffusion-weighted images, the malignant wall thickening may show patchy, inhomogeneous, or homogeneous intense diffusion restriction. Different studies have reported different apparent diffusion coefficient (ADC) values for malignant GBC. In all these studies, the ADC value was significantly lower in malignant cases than in benign cases [34-37]. An ADC value less than 1.46 ±0.45 × 10-3 mm2/s was reported by Kim et al. [35]. Using a cut-off value of 1.2 × 10-3 mm2/s along with other morphological features, sensitivity and specificity of 76.9% and 84% were reported for the identification of malignant ones [36]. There is an emerging role of diffusion-weighting in identifying the histological grade of malignancy with significantly low values associated with poor grades [38]. In addition, diffusion-weighted imaging helps identify metastatic lymph nodes, liver lesions, and omental deposits. This is demonstrated in Figure 4.
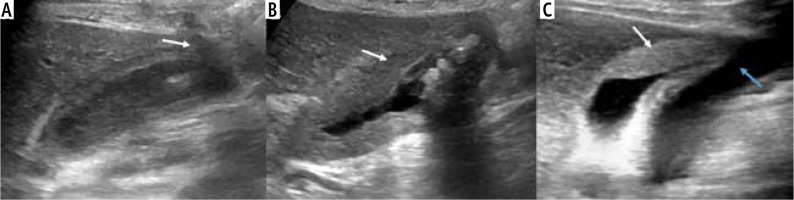
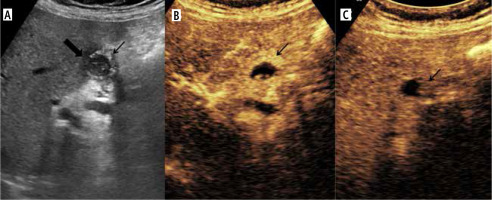
Fig. 4
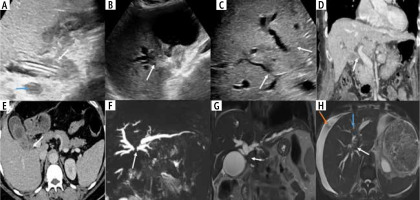
Enhancement characteristics of wall-thickening type of gallbladder cancer (GBC) in contrast enhanced ultrasound (CEUS). A) Gray-scale US image showing asymmetrical circumferential mural thickening involving the fundus and body of the gallbladder (arrow) with loss of interface with the adjacent liver parenchyma (thick arrow). B) Arterial phase image of the CEUS shows heterogeneous enhancement (arrow). C) There is early washout at 25 seconds (arrow)
Extramural extension
It is crucial to preoperatively determine the extent of involvement of the adjacent organs as surgical management varies according to the spread. For example, malignant gallbladder wall thickening can contiguously spread outside the gallbladder to involve adjoining liver parenchyma, vessels, bile ducts, the antropyloric region of the stomach, duodenum, and head of the pancreas, hepatic flexure, and transverse colon [5, 39]. CT provided 85% accuracy in the preoperative diagnosis of the locoregional extent of gallbladder cancer [40]. Amongst these, direct invasion of the liver is the most commonly seen [41]. Yoshimitsu et al. reported an accuracy of 86% in diagnosing the local extent and higher sensitivity and specificity for diagnosis of advanced lesions by CT [42]. CT’s sensitivity for detecting liver infiltration < 2 cm is 65%, which rises to as high as 100% when the infiltration is more than 2 cm [42]. The sensitivity reported for the gastrointestinal tract and pancreas involvement is 50% and 57% in the study by Ohtani et al. [43]. Infiltration into the liver parenchyma is depicted in Figure 5.
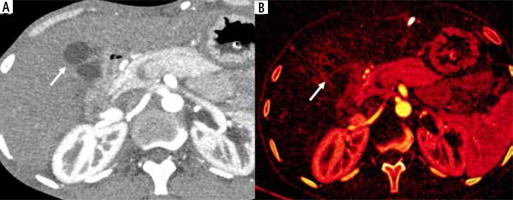
Fig. 5
Dual energy CT in wall-thickening type of gallbladder cancer (GBC). A) Axial contrast enhanced CT (CECT) image in arterial phase shows circumferential thickening of the body and fundus of the GB (arrow). B) Axial iodine overlay dual-energy CT image shows iodine uptake in the thickened areas favoring the diagnosis of malignancy (arrow)
The presence of pneumobilia or air within the gallbladder could suggest GI fistulization, mostly seen in cases of locally advanced disease [44]. Among different cholecystoenteric fistula types, the cholecystoduodenal fistula is the most common type, followed by the cholecystocolonic fistula [45]. Multidetector CT can reveal the fistulous communication and anatomical details in all three planes. There are many case reports which have reported different sites of fistulization within the gastrointestinal tract [46-48]. In most instances, the gut wall near the GBC is involved. However, circumferential involvement of the lumen mimicking primary carcinoma of the gut can also be seen [39]. Rarely, over-distension caused by gallbladder neck cancers may lead to gallbladder perforation and extramural findings that may mimic local invasion. The various patterns of extramural extension are depicted in Figure 6.
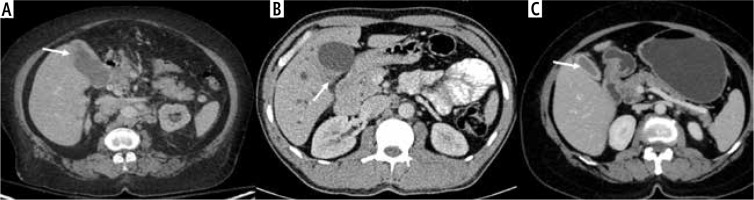
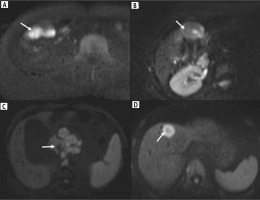
Fig. 6
Patterns of diffusion restriction in wall-thickening type of gallbladder cancer (GBC). A) The thickening involving the GB shows homogeneous intense diffuse restriction (arrow). B) Patchy diffusion restriction is present in the GB wall thickening (arrow). C) The retroperitoneal lymph node metastasis in a case of GBC was better seen on a diffusion weighted image showing intense diffusion restriction (arrow). D) The lesion in the liver shows more intense diffusion restriction in the periphery than the center, which helps to differentiate it from cholangitic abscesses (arrow)
Biliary involvement
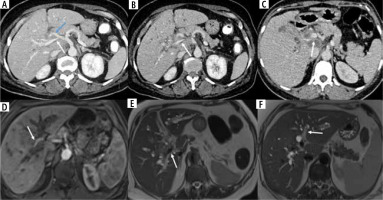
Malignant gallbladder wall thickening is known to cause varying degrees of extrahepatic and intrahepatic biliary obstruction either by contiguous infiltration of the biliary tree or by metastatic lymphadenopathy [5]. The level of biliary obstruction may vary according to the pattern of extension of gallbladder wall thickening and occur at the following levels: extrahepatic at the level of the common hepatic duct, common bile duct, head of the pancreas, pancreaticoduodenal groove, and duodenum; intrahepatic at the level of the primary confluence, secondary confluence or causing complete isolation of the segmental biliary ducts. Locoregional lymphadenopathy may cause extrinsic obstruction at any of the above-mentioned extrahepatic levels. The level and cause of obstruction are crucial in deciding the definitive/palliative treatment by external/internal biliary drainage or biliary stenting. Several studies have reported a poor prognosis in patients with biliary involvement [49]. Kondo et al. stated that the ‘‘hepatic hilum type’’, which they defined as tumor infiltration of the hepatic hilum, had a poor prognosis [50]. Cancer spread to the cystic duct has been associated with an increased incidence of lymph node and perineural invasion [51]. The patterns of biliary obstruction secondary to malignant gallbladder wall thickening are illustrated in Figure 7.
Vascular involvement
Locally advanced malignant gallbladder wall thickening may abut or encase adjacent arterial and venous structures [19]. The rate of vascular involvement increases with advanced stages and is a marker for perineural and lymphovascular invasion in histopathology [52]. Venous encasement can occur at the level of the segmental portal vein, right/left branch of the portal vein, or main portal vein. Tumor in the vein is a rare phenomenon reported in GBC, unlike hepatocellular carcinoma, with only a few case reports in the literature [53-55]. Arterial involvement can occur at the level of the common hepatic artery, right/left hepatic artery, or their segmental branches. Surgical resectability depends on the level and extent of vascular involvement. The various patterns of vascular involvement are shown in Figure 8.
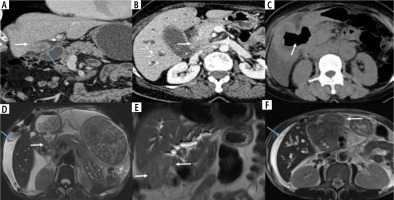
Fig. 8
Different patterns of extramural extension of wall-thickening type of gallbladder cancer (GBC). A) Coronal reformatted image showing diffuse circumferential thickening of GB with ill-defined fat planes with liver (arrow) and showing maintained fat planes with adjacent bowel loop (blue arrow). B) Asymmetrical thickening at the neck with infiltration into the duodenum and pancreas (arrow). C) Axial CECT abdomen showing extensive circumferential thickening involving the GB which is fistulizing into the 2nd part of the duodenum (arrow). D) The asymmetrical mural thickening involving the GB is extending into the antropyloric region, causing gastric outlet obstruction (arrow). Perihepatic fluid was also seen in this case (blue arrow). E) Asymmetrical mural thickening involving the GB which is infiltrating the hepatic flexure and transverse colon (arrows). F) Asymmetrical mural thickening involving the GB which is extending into the omentum (arrow). Perihepatic fluid was also seen in this case (blue arrow)
Metastasis
The thickening type of gallbladder cancer may present with distant metastasis, at times disproportionate to the degree of primary organ involvement [39, 44]. The common sites of metastasis include:
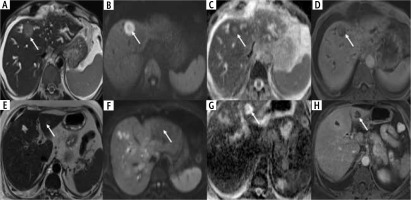
Liver: In addition to the contiguous infiltration of the liver by the malignant gallbladder wall thickening, metastatic liver lesions may also be seen, targetoid, homogeneous, or necrotic. It is essential to differentiate liver metastatic lesion from cholangitic abscess, a common mimicker. The features on multiphasic CT suggestive of abscess over metastatic disease are a combination of findings of patchy parenchymal enhancement, arterial rim enhancement, which is persistent in the portal venous phase, and perilesional hyperemia [56]. When interpreted along with other conventional MRI findings, diffusion-weighted imaging (DWI) can help in the differentiation between these two entities. On DWI, the peripheral portion of the metastasis due to high cellularity shows restriction, and the abscess is likely to show T2 shinethrough due to inflammation [57]. The patterns of liver metastatic lesions and imaging differences with cholangitic abscesses are illustrated in Figure 9.
Fig. 9
Different pattern of biliary involvement in wall-thickening type of gallbladder cancer (GBC). A) There is extension of the thickening involving the GB neck in the cystic duct (arrow) along with the presence of a few enlarged periportal lymph nodes (blue arrow). B) The GB is overdistended with asymmetrical thickening in the neck region which is extending to involve the right secondary confluence (arrow). C) Asymmetrical mural thickening involving the GB which is infiltrating the bilateral secondary confluence, causing ductal isolation (arrows). D) Coronal reformatted images show asymmetrical mural thickening involving the neck of the GB extending into the suprapancreatic CBD beyond which the CBD is dilated (arrow). E) Axial CECT abdomen showing asymmetrical thickening at the neck which extends into the cystic duct (arrow). F) Coronal reformatted magnetic resonance cholangiopancreatographic (MRCP) image showing the block at the level of the primary confluence in a case of GBC (arrow). G) Coronal T2 weighted image showing an enlarged periportal lymph node causing compression of the CHD (arrow). H) Periductal infiltration involving the primary confluence (arrow) and left secondary confluence (blue arrow) in a case of GBC. Perihepatic fluid was also seen in this case (orange arrow)
Lymph node: The various lymph nodal stations invol-ved by the GBC are defined in three levels. The 1st station includes cystic, pericholedochal, and hilar lymph nodes. Peripancreatic, periduodenal, periportal, and perihepatic lymph nodes comprise the 2nd station level, celiac, superior mesenteric artery, and para-aortic lymph nodes. The lymph nodal spread is not so predictable in GBC, and the 2nd or 3rd stations can be involved with or without the involvement of the prior station [58]. The involvement of 3rd station nodes usually precludes R0 resection. However, controversial literature exists regarding the prognosis in such patients. Kondo et al. reported a poor prognosis in para-aortic disease equivalent to distant metastasis [59]. Murakami et al. reported no significant difference in survival between patients with or without metastatic para-aortic lymph nodes among all patients with nodal involvement [60]. Hence, 3rd station lymph node involvement should not be considered an independent prognostic marker. Lymph nodal metastasis is illustrated in Figure 10.
Fig. 10
Different pattern of vascular involvement in wall-thickening type of gallbladder cancer (GBC). A) There is encasement of the main portal vein (arrow) and common hepatic artery (blue arrow) by the asymmetrical mural thickening present in the neck region of the GB. B) Multiple enlarged lymph nodes in the periportal location which are seen compressing the main portal vein (arrow). C) The main hepatic artery is compressed by multiple necrotic lymph nodes seen in periportal location (arrow). D) There is encasement of the right hepatic artery with asymmetrical thickening at the GB neck region (arrow). E) There is attenuation of the main portal vein by the asymmetrical mural thickening in the neck region of the GB (arrow). F) There is extension of thickening involving the GB along the left branch of the portal vein, causing its attenuation (arrow)
Omental and peritoneal deposits: Small omental and peritoneal deposits are rare phenomena in GBC. There is a lack of literature in this regard [61]. Yawar et al. reported a mixed pattern of involvement of the omentum, which includes both caking and nodular form [62]. GBC deposits are often seen in the hepatoduodenal ligament and lesser sac. However, they can be seen in other peritoneal spaces [61]. The nodular deposits in GBC can be subcentimetric; it is crucial to scrutinize the cross-sectional images to avoid missing them. This is shown in Figure 11.
Fig. 11
Liver metastases in wall-thickening type of gallbladder cancer (GBC). A-D) Axial T2 weighted image shows a T2 hyperintense lesion (arrow, A) in segment IVb of the liver which is showing diffusion restriction predominantly in the periphery (arrows, B and C) and is showing solid enhancement (arrow, D) suggestive of metastasis. E-H) Axial T2 weighted image shows a T2 hyperintense lesion (arrow, E) in segment IV b of the liver without diffusion restriction (arrows, F and G) and is showing a peripheral rim of enhancement (arrow, H) likely suggestive of cholangitic abscess
Others: Distant metastatic disease other than the liver and adjacent organs is rarely encountered, with only a few cases reported in the literature, to lung, bone, adrenal gland, brain, and ovaries [63-67]. These are shown in Figures 12-14. The key imaging features of the wall thickening type of GBC are summarized in Table 1.
Fig. 12
Different pattern of lymph nodal involvement in wall-thickening type of gallbladder cancer (GBC). A) Axial CECT section shows multiple enlarged necrotic regional lymph nodes in the periportal and peripancreatic region (arrow). B) Axial CECT section shows enlarged lymph nodes around the superior mesenteric artery (arrow). C, D) Axial CECT abdomen showing multiple enlarged lymph nodes in the retroperitoneum (arrow)
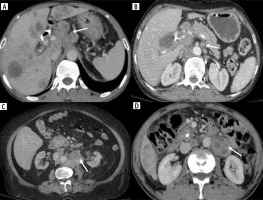
Fig. 13
Omental metastases in wall-thickening type of gallbladder cancer (GBC). A) Axial CECT abdomen shows a well-defined nodule in the omentum (arrow). B, C) Well-defined nodules of varying sizes are seen in the right paracolic gutter (arrows)
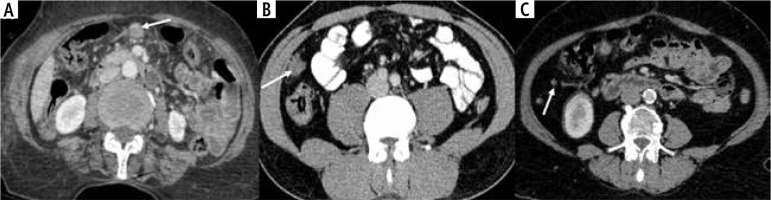
Fig. 14
Lung and adrenal metastases in wall-thickening type of gallbladder cancer (GBC). A) Multiple nodules of varying sizes are seen in the bilateral lung field along with a few atelectatic bands suggestive of metastatic nodules (arrows). B) Coronal reformatted image shows hypodense nodules in bilateral adrenal glands in a case of GBC suggestive of metastasis (arrows)
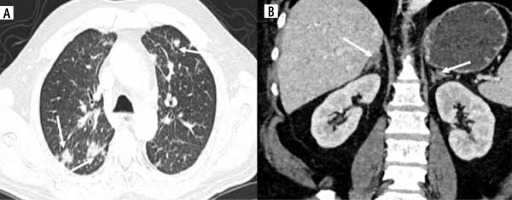
Table 1
Key imaging characteristics of wall thickening type of gallbladder cancer
Differentiating benign from malignant gallbladder wall thickening
In general, it is challenging to differentiate benign and malignant gallbladder wall thickening due to overlapping imaging features [8, 68]. Some features, including the presence of intramural echogenic foci (US) and intramural cysts, have high specificity for the diagnosis of adenomyomatosis [68]. Intramural cysts are best seen in magnetic resonance cholangiopancreatographic (MRCP) images. The presence of intramural hypodense (CT) or hyperintense (MRI) nodules involving the gallbladder diffusely suggest xanthogranulomatous cholecystitis (XGC). However, differentiation of XGC from GBC is extremely challenging due to the frequent overlap in the findings. Mural thickening in the setting of extracholecystic causes, e.g., liver and cardiac disease, is diffuse and associated with mural layering [6]. In a recent systematic review, the US features of benign and malignant gallbladder wall thickening were reported [69]. A few novel imaging techniques, including DECT and texture analysis, have recently been investigated to increase the accuracy of detection of malignant gallbladder wall thickening [70-72]. Additionally, a gallbladder reporting and data system (GB-RADS) has been proposed to stratify the risk of malignant gallbladder wall thickening in US [73]. Finally, an artificial intelligence (AI) model (GBC-net) has been proposed to differentiate benign from malignant gallbladder diseases [74]. This AI model may be investigated to identify its performance to detect malignant gallbladder wall thickening.
Conclusions
Gallbladder wall thickening presents a diagnostic dilemma, especially in geographical regions with high incidence of GBC. A multimodality approach may allow accurate characterization of gallbladder wall thickening. Novel imaging methods and techniques may further increase the accuracy of detection of malignant gallbladder wall thickening.