Imatinib mesylate, a tyrosine kinase inhibitor (TKI) and platelet-derived growth factor receptor alpha (PDGFRA), effectively inhibits cancer cell proliferation [1]. It has been established as the first-line therapy for chronic myeloid leukemia (CML), and remains a treatment option for gastrointestinal stromal tumours (GIST) and dermatofibrosarcoma protuberans (DFSP), in advanced or metastatic cases [2]. While imatinib therapy is effective, it is not devoid of side effects. Though skin hypopigmentation is a well-documented adverse effect, oral mucosa discoloration of the hard palate is also observed, albeit less frequently [3].
Here, we present three case reports detailing hard palate imatinib-induced mucosal discoloration in GIST and DFSP patients undergoing imatinib therapy, offering insights for future research into the causes of this phenomenon.
Patient 1 was a 70-year-old Polish female with intestinal GIST who presented with unknown-duration hard-palate mucosal discoloration. In 2011, she underwent segmental small bowel resection for GIST, followed by imatinib therapy. The oral examination revealed brown discoloration of the hard palate mucosa, approximately 25 mm in its highest dimension (Figure 1 A). A punch biopsy under local anaesthesia of the hyperpigmented mucosa was performed, followed by a fiberoptic examination of the upper airways revealing normal findings. The histopathological report confirmed the mucosal layer of the hard palate exhibiting fine brown spherical granules, evenly distributed. No evidence of inflammation or haemorrhage was observed. Fine, dark-brown, spherical granules were deposited within the connective tissue. No melanosis or melanocytic hyperplasia in the epithelium was seen (Figures 1 B, C). Therefore, the diagnosis of imatinib-induced hard palate mucosal hyperpigmentation was established.
Figure 1
Macroscopic view of hard palate mucosal discoloration presenting dark grey-black mucosa on the hard palate in patients 1 (A), 2 (D), and 3 (E). B, C – Histopathological images demonstrate the deposition of fine spherical brown particles. Hematoxylin-eosin staining reveals brown particle deposits in the dermis (original magnification: 400). F – Detailed description of the disease timeline in all 3 patients presented. G – Table presenting details of patient characteristics and cumulative imatinib intake. The cumulative dose and duration of imatinib intake (up to 1.08.2023 from the initiation of therapy) were calculated based on hospital systemic data
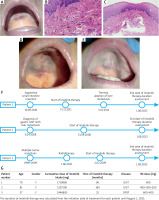
A second patient was an 81-year-old female of Polish origin with a gastric GIST with liver metastasis and hard palate mucosal discoloration, which she noticed incidentally approximately a month ago. The oral examination revealed a dark hyperpigmented lesion with a brownish colour in the hard palate mucosa, measuring 42 mm in diameter (Figure 1 D). A punch biopsy procedure under local anaesthesia was performed, followed by a routine fibre-optic examination of her upper airways revealing normal findings. The pathology report yielded results consistent with those obtained in patient 1, confirming the diagnosis of imatinib-induced hard palate mucosal discoloration.
The third patient was a 57-year-old Polish woman admitted to the clinic due to hard palate mucosal discoloration that occurred about 3 months ago. The patient had been diagnosed with an unresectable recurrence of DFSP on her right labium, treated surgically and with radiotherapy before. On oral examination, a dark brown macule on the hard palate mucosa was noticed with the highest dimension of 40 mm (Figure 1 E). After the biopsy under local anaesthesia, a fiberoptic exam found no abnormalities in the patient’s upper airways.
The pathology report confirmed imatinib-induced hard palate mucosal hyperpigmentation and was consistent with the reports of patients 1 and 2. Details on the disease timeline of all patients are presented in Figure 1 F. While the literature suggests possible mechanisms for imatinib-induced mucosal discoloration [4, 5], the exact causes remain poorly studied, necessitating further investigation. Each case report provides valuable insights for guiding future research into this phenomenon (Table 1). The findings suggest a potential association between hard palate mucosal discoloration and the total dose of imatinib intake considering the markedly high cumulative doses observed in these cases (Figure 1 G).
Table 1
List of the research studies on imatinib-induced oral mucosa discoloration
The findings align with data presented in some publications [6–8] particularly Oliveira et al. [8] who have conducted research on hyperpigmentation occurrence in patients taking imatinib with only 4 patients undergoing a punch biopsy of the hard palate mucosa. Their study indicates that factors such as the duration of imatinib intake exceeding 72 months, male sex, African descent, and age over 49 have an impact on hard palate mucosal discoloration. However, in their research study, one of the independent variables was the duration of imatinib usage itself, without consideration of the cumulative dose of imatinib intake. Generally, most patients with GIST or CML take the standard dose of imatinib, which is 400 mg daily. Nevertheless, due to some intolerable side effects, such as diarrhoea or skin rashes, the dosage is sometimes adjusted reduced [6]. On the other hand, in certain cases, especially those with disease progression on standard-dose therapy or patients whose tumour is associated with an exon 9 mutation in KIT, the initial treatment of 800 mg/day is recommended [9]. Moreover, only 4 patients in Oliveira’s research underwent a punch biopsy of the hard palate mucosa. Therefore, while the possibility of a different diagnosis is low, it remains conceivable. The limitations of these case report studies include their cross sectional design, where the occurrence of mucosal discoloration was assessed at a single point in time. However, although determining the precise dose leading to the hyperpigmentation occurrence is challenging, these findings provide a foundation for future prospective studies on imatinib.
In summary, the presented case reports imply a potential correlation between hard palate mucosal discoloration and the total dose of imatinib intake. Moreover, to establish a definitive diagnosis of imatinib-induced mucosal hyperpigmentation, a biopsy and pathological examination of hyperpigmented mucosa are required in every case as the differential diagnosis of melanoma should always be ruled out. The oral examination should be accompanied by an endoscopic exam at that time. Regardless of the hard palate hyperpigmentation, patients should have follow-up visits in compliance with their main disease treatment guidelines. Although no treatment is required for the mucosal discoloration of the hard palate due to its indolent and benign nature, further research needs to be conducted on a larger group of patients as the pathogenesis of imatinib-induced hyperpigmentation is still not well studied. Additionally, more education about possible mucosal discoloration caused by imatinib and other medications should be implemented, especially among ENT doctors, maxillofacial surgeons, and dentists.








