Introduction
First cases of the pediatric multisystem inflammatory syndrome associated with SARS-CoV-2 infection (MIS-C) were reported in EU/EEA countries and the UK in spring 2020 [1-5]. In Poland, we did not observe them until autumn. According to the European Centre for Disease Prevention and Control (ECDC), in May 2020, Poland was still before the peak of the epidemic. From March 13, 2020, people were advised to self-isolate, and schools and kindergartens were closed. As pediatricians, we observed a marked decrease in infections in children [6]. The number of children consulted in an emergency room and hospitalized was much lower compared to previous months. Recent studies revealed that children are at a similar risk of COVID-19 infection to the general population. In other countries, MIS-C was reported relatively late during the waning tail of the first epidemic curve [7]. Countries with large outbreaks of SARS-CoV-2 (France, Italy, Spain, UK, US) have seen the occurrence of cases of pediatric inflammatory MIS-C in the late stages of the first wave of the COVID-19 pandemic [8-13]. Verdoni and colleagues, in an article published in The Lancet, described a recent outbreak of Kawasaki disease (KD) in Italy [1]. Clinicians in Europe and the United States have identified clusters of similar cases [14-17]. The observed epidemic of MIS-C disease in many countries is attributed to the SARS-CoV-2 pandemic. However, it has become evident that shock, gastrointestinal symptoms, and coagulopathy, which are rarely seen in classic KD, are prominent features of this unique syndrome. The pathophysiology of MIS-C and the T and B cell response to SARS-CoV-2 infection remains poorly understood. Peripheral natural killer (NK) cells are depleted and exhibit an exhausted phenotype in patients with severe COVID-19 [14]. Many analyses suggest that extravasation of T and NK lymphocytes and activation and chemotaxis of neutrophils and nonclassical monocytes likely contribute to the underlying disease pathogenesis [14]. In MIS-C, there is observed an elevated level of chemokines that recruit NK cells and T cells from the circulation (CCL19, CXCL10, and CDCP1) [15-17]. Alternatively, perturbations in hematopoiesis cannot be ruled out entirely.
This study aimed to define peripheral blood immune features and the clinical evolution of symptoms in patients with MIS-C.
Material and methods
Between April 1 and May 15, 2021, at the Infectious Diseases Department in St. Joseph’s Children’s Hospital in Poznan (Poland), eight children were hospitalized with an inflammatory disease similar to KD. We analyzed only patients with fever without an identified source and no response to antibiotic therapy who fulfilled diagnostic criteria for MIS-C [18]. On admission, laboratory tests including complete blood count, C-reactive protein (CRP), potassium, sodium, and transaminase levels, urinalysis, blood and urine cultures, chest X-ray, and abdominal ultrasound were performed. Children underwent cardiology consultation. KD was diagnosed in patients with fever lasting five days or longer accompanied by four or more of the following clinical criteria: bilateral nonexudative conjunctivitis, erythema of the lips and oral mucosa, changes in the extremities, rash, and cervical lymphadenopathy [6].
MIS-C case definition criteria proposed by the Royal College of Pediatrics and Child Health are described be- low [18]:
A child is presenting with persistent fever, inflammation (neutrophilia, elevated CRP, and lymphopenia), and evidence of single or multi-organ dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurological disorder) with other additional clinical, laboratory, or imaging and electrocardiogram (ECG) features.
Children fulfilling full or partial criteria for KD may be included.
Exclusion of any other microbial cause, including bacterial sepsis, staphylococcal or streptococcal shock syndromes, infections associated with myocarditis such as enterovirus.
SARS-CoV-2 PCR testing positive or negative.
Flow cytometry analysis was used for the detection of lymphocyte subsets. Samples of EDTA anticoagulated peripheral blood (2 ml) were obtained from all patients on admission. CD4+, CD8+ T-cell, and B-cell counts (cells/µl) were measured using multiple-color flow cytometry. Lymphocyte subpopulations were characterized by combining monoclonal antibodies for T, B, and NK cells:
– T cells: (CD45+, CD3+), (CD45+, CD3+, CD4+), (CD45+, CD3+, CD8+)
Naive Th CD4+RA+CD27+
Central memory Th CD4+RA–CD27+
Effector memory Th RA–CD27–
Terminally diff. memory Th CD4+RA+CD27–
Recent thymic emigrants Th CD4+CD31+ RA+
Treg CD4+CD25+CD127–
Naive Tc CD8+CD127+CD27+
Central memory CD8RA-CD197+CD27
Effector memory Tc CD8+RA–CD197–CD27–
Terminally diff. memory Tc CD8+RA+CD197–CD27–
– B cells: (CD45+, CD19+)
CD27+ Memory
CD27– Non-memory
CD27+sIgD+ Non-Switched-Mem
CD27–sIgD+ Naive
CD27+sIgD– Switched-Mem
CD38hi sIgMhi Transitional
CD38lo CD21lo Activated
CD21lo Immature
– NK cells: (CD45+, CD56+, CD3–).
The Bioethical Committee of Poznan University of Medical Sciences approved the study. Informed consent was obtained from all participants’ guardians. Blood was collected as a part of routine tests during hospitalization – no additional procedures were performed.
Results
Of the eight hospitalized children, seven agreed to take part in the study. There were three girls and four boys, aged from four to nine years. They all presented with fever not responding to antibiotic treatment, irritability, and abdominal pain. Inflammatory markers were elevated: neutrophilia, significantly increased CRP, ferritin levels, raised troponin and BNP levels. Echo and ECG were performed in all patients – in two coronary artery dilatation was found. One patient had markedly decreased heart contractility (ejection fraction 24%) and he was diagnosed with dilated cardiomyopathy.
Three patients were diagnosed with pneumonia. Typical findings on abdominal ultrasound were ascites and hepatosplenomegaly. Details are presented in Table 1. One patient underwent an appendectomy before being diagnosed with MIS-C.
Table 1
Demographics, clinical findings, imaging findings, treatment, and outcome
There was no COVID-19 history in one patient, one patient had a family history of COVID-19 four weeks earlier, and one five months earlier. Three patients had asymptomatic or mildly symptomatic COVID-19 three/four weeks earlier. None of the tested children was found to be positive for SARS-CoV-2 by PCR. All patients were positive for IgG SARS-CoV-2 antibodies. The median level of antibodies was 198.27 BAU/ml (positive ≥ 7.1 BAU/ml). The lowest level was 14 BAU/ml (2 ULN), detected in a patient who had exposure to SARS-CoV-2 five months earlier.
Coinfection with other pathogens has been investigated, and no pathogens have been detected: blood cultures were negative, and there were no serological markers of EBV infection.
All patients received empiric antibiotic therapy – two patients received only one dose of ceftriaxone, while the rest received a full 7-day course. All children fulfilled the MIS-C case definition criteria proposed by the Royal College of Pediatrics and Child Health. All the children received the following treatment: intravenous immunoglobulin (IVIG) 1-2 g/kg given over 12 h, methylprednisolone 2 mg/kg/day for five days, and a low dose of aspirin (3-5 mg/kg max. 75 mg).
All patients survived, and their health status improved with time. A rapid response was observed after introduction of the treatment (IVIG and methylprednisolone), fever and abdominal pain subsided, and inflammatory markers and lymphocyte level return to normal. One child had severe cardiac dysfunction that required vasopressor treatment. At the follow-up visit after six weeks all patients presented with good health. One patient experienced permanent heart dysfunction and was unable to return to daily activities.
Lymphocyte subsets in peripheral blood of patients
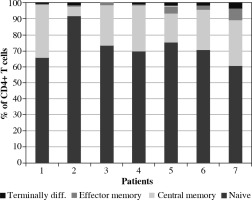
Lymphopenia was detected in all patients. A marked decrease in both CD4+ and CD8+ T cells was observed, with levels much below age-matched reference ranges (Table 2). In CD4+ T cells, majority subsets were: naive cells, CD45RA+ cells, recent thymic emigrants. The percentages of T regulatory cells (Tregs) ranged from 2.9% to 8.2%. Details are presented in Figure 1.
Table 2
Lymphocyte subsets in peripheral blood of patients with MIS-C
Fig. 1
Lymphocyte T CD4 subsets (terminally differentiated, effector memory, central memory, naive) in peripheral blood of patients with MIS-C
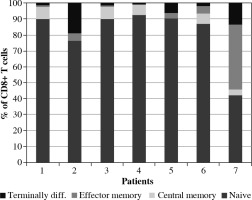
The majority (6/7) of the analyzed patients had a higher CD4+/CD8+ T cell ratio than average values. Apart from low CD4+ levels, also a marked reduction in CD8+ T cells was detected. The majority of the T cells were naive cells (Figs. 1, 2). B lymphocytes were within the normal range – the majority were non-memory cells. Plasmablasts (PBs) were detected only in one patient. The number of NK cells was low in all patients.
Discussion
The clinical symptoms and laboratory results of the observed children in MIS-C resemble those of children with KD or adult patients with acute COVID-19. The laboratory results such as high inflammatory markers, lymphopenia, neutrophilia, anemia, low platelets, altered coagulation, and high cardiac markers present in our patients are typical for adult patients with active severe COVID-19 [19, 20].
Upon recovery from COVID-19, results return to normal. Complications of COVID-19 and so-called “long COVID” seen in adults weeks after primary infection are not accompanied by high inflammatory marker levels [21].
During acute viral infections or after vaccination, PB responses are transiently detectable in the blood and correlate with CD4+ T cell responses [22, 23].
In the six out of seven observed patients, PBs were not detected. So anti-spike antibodies cannot be related to the mechanism of MIS-C development. This finding is significant regarding the vaccination which is now offered to adolescents. The risk of developing MIS-C cannot be established based on the IgG level. None of the children had severe COVID-19 in the past – three of them had no symptoms or mild symptoms – and did not need medical help with this. It appears that MIS-C is an inflammatory immune-mediated SARS-CoV-2 postinfectious process triggered by the virus rather than the effect of the prolonged viral infection.
Lymphopenia was observed in all our patients. Transient lymphopenia is quite common at the beginning of viral infections, but it resolves over time [23, 24]. Low lymphocyte levels, including reduced CD4+ and CD8+ T cells, are also seen in patients with acute COVID-19 – both children and adults [25, 26]. By contrast, COVID-19 patients had high polymorphonuclear leukocyte (PMN) counts; monocyte, eosinophil, and basophil counts were normal [25-27]. This is also commonly seen in both COVID-19 and MIS-C patients [28, 29]. A higher total T cell count, including both CD4+ and CD8+, is a predictor of less severe disease and more favorable clinical outcome. Robust CD4+ T cell activation, lack of cTFH cells with proliferating effector or exhausted CD8+ T cells, and PB involvement were associated with more severe disease [25, 26, 30]. Low levels of CD4+ in COVID-19 patients can be explained by extravasation and emigration to the site of infection. Effusions composed of T cells were detected in the lungs of patients with severe COVID-19 [31]. But this mechanism cannot explain lymphopenia in patients with mild COVID-19 or children. There are several causes of the observed lymphopenia in severe COVID-19 patients. SARS-CoV-2 can induce pyroptosis in lymphocytes via induction of the NLRP3 inflammasome [32]. Massive lymphocyte death can also be attributed to high levels of interleukin 6 (IL-6) as well as Fas-FasL interactions [33]. COVID-19 infection can result in exhaustion of T cells. Markers of T cell exhaustion – programmed cell death protein 1 (PD-1), T cell immunoglobulin and mucin domain 3 (Tim-3) – were found on the surface of CD4+ and CD8+ T cells from COVID-19 patients [34]. High levels of CXCL10 and CCL2 can suppress the development of hematopoietic precursor cells. T cell count returns to normal in recovered COVID patients [25].
The average CD4+/CD8+ ratio in healthy patients is not well defined. Ratios between 1.5 and 2.5 are typically regarded as normal [25]. The CD4+/CD8+ ratio was higher than usual in the observed group – a profound decrease in CD8+ cells caused it.
This was also found in other studies on adults with COVID-19 [34]. In MIS-C patients, the frequency of CD8+ T cells was lower compared to adults with COVID-19. In some studies in severe COVID-19 elevated levels of CD8+ T cells persist over time [22]. Low CD8 levels were observed in COVID-19 patients with a bad prognosis [21]. In HIV infected patients loss of CD4+ T cells is accompanied with an increase in CD8+ T cells, which compensates for the loss of CD4+ T cells [23].
Analyzing T cells subsets, we detected that the frequency of naive CD4+ and CD8+ T cells was higher than the reference range – this is typical for pediatric patients. For both CD4+ and CD8+ T cells, the frequency of central memory (CM, CD45RA–CD27+CCR7+) and effector memory 1 (EM1, CD45RA–CD27+CCR7–) was lower than the reference range. Similar findings were described by Vella et al. [28]. Terminally differentiated effector memory T cells (EMRA, CD45RA+ CD27- CCR7-) were present at a lower frequency in our pediatric cohorts. The naive, effector and central memory subset distributions were not different between the pediatric COVID-19 and MIS-C.
The period for peak CD8+ or CD4+ T cell responses during many acute viral infections and the window for PB detection in peripheral blood are usually short. A prolonged period of peak immune responses (CD8+, CD4+ T cell activation, and PB presence) observed during COVID-19 suggests a failure to appropriately down-regulate reactions in some patients. In the analyzed group B, cells were usually within the normal range. This was also noted in other studies [27-29]. Plasmablasts were not detected in our patients. A notable feature of some patients was a decrease in memory cells, effector memory cells, and terminally differentiated memory cells. In a study by Vella et al., they observed prolonged plasmablast responses and a profile of B-cell subsets similar to that in MIS-C and COVID-19 patients [28]. In this American study on 14 patients, 86% were PCR positive up to a cycle threshold of 45. They suggested that MIS-C can be caused by continued activation of the adaptive immune response, driven by persisting antigen in the later stage of a poorly controlled primary infection. Persistent antigen stimulation cannot explain all cases of MIS-C. Their data suggested that PB responses were altered compared to pediatric COVID-19. Prolonged PB presence was not detected in adults weeks after COVID-19. Elevated PB frequencies were surprising given that MIS-C occurs weeks after SARS-CoV-2 infection. In our group a boy with the lowest level of antibodies had a history of positive SARS-CoV-2 PCR six months earlier. But unfortunately, he was also the most affected patient. He developed severe cardiac complications.
Anemia is a prevalent finding in patients with severe COVID-19. It was previously reported that COVID-19-related anemia showed several RBC shape abnormalities [23]. Erythrocyte deformability may be involved in COVID-19 severity, causing tissue hypoxia. In three patients, we detected abnormalities in blood smear – the formation of erythrocyte “rouleaux”. These morphological changes may contribute to the microvascular thrombosis typical of COVID-19. In all patients with MIS-C, D-dimers were elevated.
Vascular inflammation, particularly endotheliitis, can explain many of the organ changes observed in patients with MIS-C and in KD patients. Similar to observational studies, our analysis shows that MIS-C is of higher preponderance in the age group of 5-14 years. This age distribution is different from that of classical KD, which is common in children of younger age. Lymphopenia and low platelets are not commonly seen in KD [35]. The immune profile of KD patients is very diverse. They do not have one immune trait. In a study published by Burns et al., CD4+ T cells were in the normal and low range; some patients showed significant CD8+ lymphocytosis [36].
The weak aspect of this study is the small group of patients. It was difficult to enroll more patients over a short period of time. This can be explained by the fact that MIS-C is a rare clinical entity; blood samples had to be collected and immediately tested before introduction of the treatment. It is not uncommon for MIS-C studies to be performed on such a small group of patients. These preliminary results will be further developed when new MIS-C patients are treated.
The immune profile of the studied patients differs from that of children with KD, but it is similar to that of adults with severe COVID-19. Immune exhaustion can be a possible mechanism of MIS-C. The proposed explanation is profound lymphopenia caused by SARS-CoV-2 infection – which persists for weeks, leading to uncontrolled inflammation and immune dysregulation. Our data suggest that MIS-C is an inflammatory disease similar to severe COVID-19 in adults. This study helps to understand the immune status of MIS-C patients before initiation of treatment and gives a hint of possible triggering mechanisms.
There is a question of whether lymphopenia observed in MIS-C is a consequence of lymphopenia caused by SARS-CoV-2. According to our data, the activation-induced cell death (AICD) mechanism may explain both lymphopenia as well as depletion of activated T cells and suppression of the B cell response.
In COVID-19 patients the T cell level returns to normal after the second week of the disease. Our data suggest that in children prolonged lymphopenia after COVID-19 can be a practical marker for possible MIS-C alert. If there is a continuum from lymphopenia to MIS-C, there is a room for screening and prevention. Further studies are needed to determine whether steroid treatment introduced in a child with prolonged lymphopenia could stop the inflammatory process.
Conclusions
Lymphopenia and functional aberrations in T-cell subpopulations were detected in all patients with MIS-C.
Patients with MIS-C displayed reduced CD4+ and CD8+ T cell levels.
The CD4+/CD8+ratio was increased in all patients.
Predominance of naive CD4+ and CD8+ cells and suppression of terminally differentiated B and T cells were observed. The observed low activation of memory T cells usually occurs in viral infections.
Possible mechanism of MIS-C – prolonged lymphopenia caused by viral infection leads to an inflammatory response.