Arterial hypertension and its consequences
Arterial hypertension (AH) is a disease of civilization that affects the entire society to a greater and greater extent – regardless of gender, age, or level of affluence. This disease is diagnosed differently in different age groups – in developmental age, it is defined as systolic and/or diastolic blood pressure persistently at least the 95th percentile for sex, age, and height measured on at least three separate occasions, whereas in adolescents 16 years old and older the definition is not based on the 95th percentile but on the absolute cut-off point used for adults, which in Europe is 140/90 mmHg [1]. Most obviously, AH is one of the most significant risk factors for the development of cardiovascular disease, chronic kidney disease, and, therefore, premature mortality. The pressure exerted by the circulating fluid in the vessels causes damage to them and – through various mechanisms – leads to their remodeling. This applies to small, medium, and large vessels. In addition, it has further consequences for the organs they nourish. In the case of the kidneys, the glomerular filtration is damaged, causing microalbuminuria; the damage to the eye vessels leads to retinopathy; in the case of the aorta and large arteries, such as the carotid arteries, remodeling caused by hypertension predisposes to arteriosclerosis, and then, based on these changes, to accelerated plaque development (atherosclerosis) [2]. Changes in the coronary arteries and the aorta also significantly affect the heart, causing left ventricular hypertrophy, heart failure, and myocardial ischemia.
Epidemiology of arterial hypertension
Due to the aforementioned effects of the disease, the increasing prevalence of hypertension in society observed for many years is a very worrying phenomenon. Every year more and more cases of this disease are diagnosed. It is estimated that around 1.39 billion people suffer from arterial hypertension worldwide [3]. This is especially dangerous in the case of the population of children and adolescents, where it has been a marginalized problem for many years, whereas, in fact, developing the disease at such an early stage shortens the estimated life expectancy relatively the most. Meanwhile, along with the growing problem of the increasingly frequent and higher degree of obesity in patients under 18 years of age, the increase in the prevalence of arterial hypertension goes hand in hand. It is estimated that approximately 4% of children are affected by AH [4]. Obviously, the epidemiology of AH differs in various age groups. It is extremely rare in neonates and infants, reaching up to 10% in the second decade of life. Traditionally, secondary hypertension was considered the leading cause of blood pressure elevation in developmental age. In contrast, novel analyses suggest that primary hypertension (PH) accounts for half of all cases of elevated blood pressure in pediatrics [5].
Hypotheses for the development of primary hypertension
Several concepts have been proposed to explain how primary hypertension develops. First, it is caused by two basic mechanisms – increased cardiac output exerting more than necessary hydrostatic pressure on the vessel walls and increased vascular resistance opposing the blood pressing against them. These phenomena can occur separately or together, follow each other or develop simultaneously, and each has been studied in detail by researchers worldwide for many years [6]. Because many factors influence the development of properly functioning mechanisms regulating blood pressure, each of them must be considered as a potential source of control disorders, predisposing to blood pressure increase and the development of hypertension.
First, there are genetic factors. To date, more than 30 genes and over 1400 single-nucleotide polymorphisms (SNPs) have been identified as associated with hypertension [7]. Many of them have widened our understanding of the pathophysiological process of developing PH, and, moreover, they can be potential targets for more personalized and effective treatment of this condition. The next group is kidney mechanisms – according to Guyton’s hypothesis – patients with PH (at least those who are “salt-sensitive”) are characterized by excessive sodium reuptake in the distal convoluted tubule (DCT), thus leading to “pressure natriuresis” [8]. Moreover, kidneys are a source of renin – the first component of the renin-angiotensin-aldosterone system (RAAS), and other blood pressure-influencing mediators such as prostaglandins, medullipins, and adrenomedullin. Another link between renal disorders and hypertension is uric acid, elevated levels of which researchers have associated with the development of PH. The conducted studies confirmed that both symptomatic and asymptomatic hyperuricemia is a risk factor for the development of cardiovascular diseases [9].
There is a number of other mechanisms and concepts which attempt to explain the origin of PH. The following ideas are worth mentioning as possible triggers for PH in the pediatric population: endocrinological disturbances (including local and systemic RAAS overactivity), increased sympathetic tonus, disturbed endothelial function (with overproduction of endothelin and nitric oxide deficiency), obesity-related mechanisms (leptin resistance, overproduction of angiotensinogen along with other adipokines, overactivity of RAAS or dipeptidyl peptidase 4), and vitamin D deficiency. Three relatively new concepts are now being intensively studied: firstly, the phenomenon of early biological aging (including early vascular aging – EVA); secondly, the concept of hyperkinetic circulation postulated by Stevo Julius and reinvented in recent years; finally, the last two decades have brought numerous findings on the impact of immunological disturbances in the pathogenesis of PH [10].
Immunological mechanisms of the development of primary hypertension
The concept of involving the immune system in the development of primary hypertension first appeared in the 1960s. It began with a study by Okuda and Grollmann, in which they observed that implanting lymphocytes from rats with a kidney infarction in healthy individuals led to an increase in their blood pressure [11, 12]. Despite significant findings in the following years, including the discovery of the perivascular infiltration of leukocytes in patients with PH, the idea of further exploring this role was dropped for a long time. Recently, we can observe renewed interest in studying the importance of the immune system in the development of primary hypertension [13, 14]. The latest research promotes the thesis of the role of salt and shear stress as inducers of inflammation by differentiating naive T lymphocytes towards the Th17 phenotype, increased production of proinflammatory cytokines (such as interleukin (IL)-17, increasing the aforementioned sodium reuptake in DCT), and reactive oxygen species (ROS), inhibition of macrophage activation and regulatory T lymphocytes (Tregs) [15, 16]. Of note, both innate and adaptive immune cells play a role in the development of PH [16].
Another example of the significant role of immune cells is the participation of cytotoxic T lymphocytes in the promotion of PH development, as evidenced by the discovery of accumulated CD4+ and CD8+ populations in the kidneys of hypertensive mice. In addition, evidence was found for the effect of the CD8+ population on the upregulation of the sodium-chloride cotransporter and enhanced sodium ions and water reabsorption in kidneys [17].
Immunological mechanisms of arterial damage in the course of primary hypertension
One of the most important forms of hypertension-related organ damage (HMOD) in children with PH, which is often the cause or accelerator of other complications, is arterial damage. The studies carried out so far have produced several important findings, such as an increase in the number of values – carotid artery diameter, carotid intima-media thickness (cIMT), and arterial stiffness indices in adolescents with PH [10]. The latest pediatric guidelines of the European Society of Hypertension (ESH) noted the importance of these phenomena by introducing the measurement of pulse wave velocity (PWV) and cIMT as additional assessments of HMOD [1]. It is worth noting that recent studies have confirmed a correlation of increased values of aortic PWV and cIMT with hard endpoints in adult patients [18, 19].
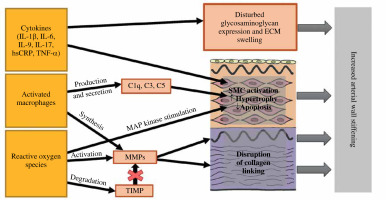
Despite a number of studies, the exact mechanism of early arterial damage (early vascular aging – EVA) in children with PH still needs to be fully discovered. Remodeling of the arterial wall, including increasing its thickness with a compensatory increase in the amount of collagen fibers resulting in a decrease in elasticity and an abnormal structure of the vascular muscle layer, is a phenomenon that has been observed in pediatric PH patients. The imaging tests of vessels make it possible to estimate its progress and the risk of developing cardiovascular complications. Due to the fact that this phenomenon is reversible, it also constitutes a good marker of recovery and the effectiveness of therapy. Nevertheless, the exact nature of the successive stages of change in the artery wall structure still needs to be confirmed. Among the proposed mechanisms, immune disorders have been suggested [20]. Inflammation was one of the most important immunological processes that showed great promise in investigating the causes of vascular damage. The related activation of immune cells leads, inter alia, to the increased production of substances with proven tissue-damaging effects – cytokines, ROS, metalloproteinases (MMPs) or transforming growth factor β (TGF-β). Secondly, damage to the epithelium and dysfunction of the pressure regulating mechanisms cause the migration of activated immune cells into the vascular wall, leading to an intense inflammatory reaction in this localization [16]. The study of which cell populations and their secreted substances are involved in this process seems to be a good starting point for a better understanding of the EVA phenomenon.
A concept that has been intensively explored in recent years is the role of the complement system in the process of vascular remodeling. Firstly, it should be noted that it may be involved at various stages of the same mechanism. For example, the C3 and C3a components activate the extracellular signal-regulated kinase 1/2 (ERK1/2) cascade and stimulate the proliferation of macrophages and smooth muscle cells (SMC) in the vessels [21]. Secondly, C1q released from macrophages, together with the SMC-derived C1r/s component, forms the C1 complex, which activates the β-catenin signaling pathway and induces myocyte proliferation [22]. Moreover, the above-mentioned C3 component and C5, also secreted by macrophages, induce myofibroblasts’ differentiation [23]. From all the examples, it can be argued that further exploring the mechanisms involving the complement system may bring new knowledge about hypertension-dependent vascular remodeling.
The proposed immunological mechanisms of arterial wall damage are presented in Figure 1.
Experimental studies
In 2016, Itani et al. published the results of a study investigating the activation and tissue penetration of T lymphocytes in humanized mice with angiotensin II (Ang II) induced hypertension [24]. Apart from kidney and lymph node changes, the most important observations concerned the aorta. Induction of PH with Ang II resulted in a noticeable increase in the number of CD45RO+ memory T cells and CD4+ memory T cells. Moreover, the use of protective doses of hydralazine and hydrochlorothiazide resulted in a decrease in the number of all memory T cells (including the CD4+ population) but also a significant decrease in the accumulated CD3+ and CD8+ T cells. Both the effect of the increase and the decrease in the number of T cells caused by pharmacotherapy turned out to be independent of Ang II itself and resulted from changes in the magnitude of hydrostatic pressure in arterial blood. In addition, the observations made during the study promoted the authors’ earlier idea that T-cell activation was one of the important enhancers of EVA [25].
As it has been proven in many studies, large arteries, especially the aorta, are subject to damage and remodeling processes in the course of PH at a very early stage for many reasons. This is why Moore et al. studied the influence of macrophages on changes in the structure of the aortic wall [26]. The study used mice whose blood pressure was increased with an infusion of Ang II. The most important observation confirmed that macrophages accumulate in the aorta and reach the M2 polarization state. Based on other animal models used in studying tissue remodeling processes in which M2 macrophages were found [27-29], this would support the hypothesis that this particular cell population may influence the differentiation of fibroblasts towards collagen-producing myofibroblasts. The participation of mediators secreted by M2 macrophages – TGF-β, platelet-derived growth factor, MMPs, tissue inhibitor of metalloproteinase-1 (TIMP-1) or fibronectin – in this process is considered. An additional mechanism from an earlier in vitro study that concerned M2 macrophage stimulation of vascular smooth muscle cell proliferation was also taken into consideration [30].
Another interesting study conducted in mice with Ang II-induced hypertension by Nosalski et al. analyzed the role of T cells in the process of vascular injury at the molecular level. Researchers observed that of all tagged T cell-derived microRNAs (miRs), microRNA-214 (miR-214) is of the greatest importance [31]. The deletion of miR-214 prevented the development of perivascular fibrosis, arterial stiffness, and hydroxyproline accumulation without affecting blood pressure. Moreover, miR-214–/– mice showed a significantly less intense endothelial damage process with reduced ROS secretion and profibrotic cytokines – IL-17, tumor necrosis factor α (TNF-α), IL-9, and interferon γ (IFN-γ). This was confirmed by the PWV measurements, which showed reduced arterial stiffness in miR-214–/– individuals [31].
Studies in adults
A study carried out by Barbaro et al. in patients with resistant hypertension analyzed the relationship between a number of inflammatory mediators suspected in previous publications to induce the development of the PH itself and its vascular complications. Interleukins IL-6, IL-10, and IL-1β, along with TNF-α and high-sensitivity C-reactive protein (hs-CRP), were taken into account. With all the mentioned particles in a manner characterized with arterial stiffness, determined by the PWV value, only IL-1β concentration correlated significantly [32]. It is an interesting fact due to the earlier study of Mahmud and Feely, which also investigated the links between inflammatory mediators and arterial stiffness, but in patients with PH. They observed that the remaining molecules, namely IL-6, TNF-α, and hs-CRP, showed a positive correlation with arterial stiffness parameters [33]. This comparison confirms that the inflammation and mediators separated in it modulate the remodeling process of arterial vessels, and more can be looked at for differences in the distribution of individual markers depending on the form of hypertension.
The study by Kontaraki et al. focused on the molecular mechanisms linking HMOD and activation of T cells in the course of PH. Using the observations from previous studies on mice, it was decided to examine the concentration of miR-9 and miR-126 in patients with PH [34]. These substances have been shown to be important in vascular and left ventricular muscle remodeling processes, especially miR-126, which is involved in transmitting signals regulating endothelial repair and plays a protective anti-atherosclerotic role during vascular remodeling. The study showed that the examined patients were characterized by increased expression of both miRs, i.e., miR-9 and miR-126, in peripheral blood mononuclear cells (PBMCs). The marked levels of miRs were related to the HMOD parameters – LV mass index and 24 h pulse pressure (PP) – therefore, they were incorporated as potential markers of cardiovascular events and HMOD.
Studies in children
It must be said clearly that there are few publications examining the role of the immune system in the development of HMOD, focusing on the pediatric population. In our study carried out on pediatric patients, we were able to indirectly link the elevated parameters of the complete blood count – neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and mean platelet volume (MPV), associated in studies on adult patients with subclinical inflammation – with vascular HMOD indicating their remodeling: significantly faster aortic and carotid arteries’ pulse wave velocity, thicker intima-media and increased maximum diameter of the common carotid artery [12]. It was not an original idea to associate the inflammatory process with the development of primary hypertension or PH itself with vascular remodeling, but it was the first study to suggest that the intramural inflammatory process in primary hypertension affects many arteries already at the developmental age.
In another study, Gackowska et al. investigated the effect of loss of the CD31 receptor in the CD4+ and CD8+ T-cell populations in children with PH [35]. The authors were able to link this phenomenon directly with arterial stiffness. In the performed measurements, the number of CD4+/CD31– T-cells significantly correlated positively with the indirect parameters of arterial stiffness – PWV, central systolic blood pressure (cSBP), and central pulse pressure (cPP). Based on previous experimental work, it was suspected that the mechanism directly linking changes in the CD31– to CD31+ cell ratio with the process of vascular wall damage and repair is disruption of the cytokine expression pathway, but no conclusive evidence was found [35].
The subsequent work by the same authors analyzed the influence of the distribution of regulatory T lymphocytes on the intensity of PH and the development of HMOD [36]. There was described a phenomenon that could support the hypothesis that PH in children is associated with abnormal Treg cells homeostasis. It has been observed that the elevated frequencies of CD45RA– memory/activated Tregs, CD31– Tregs, and CD45RA+ CD31– mature naive Tregs positively correlated with arterial stiffness and PWV. This phenomenon and the effects of CD31 receptor loss described previously add up to evidence strongly suggesting the involvement of a malfunctioning thymus and certain subsets of circulating T cells in the development of PH in children and a possible cause of vascular damage.
Another study by Niemirska et al. links the vascular injury process with increased secretion of matrix metalloproteinase 9 (MMP-9) and TIMP-1 [37]. These substances are secreted by a number of cells – endothelial cells, fibroblasts, smooth muscle, and infiltrating inflammatory cells. In a properly functioning organism, their activity remains in balance, controlling the deposition of the extracellular matrix (ECM) and protecting the vascular wall against the excessive synthesis of collagen fibers and/or their insufficient degradation. However, under conditions of inflammation, especially inflammatory cells infiltrating the vessel wall begin to overproduce both substances – MMP-9 and TIMP-1 – disrupting this balance and leading to wall hypertrophy and excessive collagen deposition at the expense of loss of elasticity. Interestingly, the authors managed to confirm the relationship between hypertension and high levels of MMP-9 and TIMP-1 only in boys with hypertension. This phenomenon was not associated with BMI but was related to the occurrence of metabolic syndrome in the studied patients. Moreover, only in the case of TIMP-1 was there a strong correlation between its increased plasma concentration and the increase in the indirect parameters of arterial stiffness. The observed discrepancies may indicate a relationship between the activity of metalloproteinases and the activity of sex hormones or adipose tissue and are an interesting starting point for further research [37].
The same group of authors analyzed the relationship between peripheral blood leukocyte adiponectin receptors, the presence of primary hypertension, and HMOD [38]. The patients with PH whose leukocytes showed expression of adiponectin receptors had higher blood pressure and greater carotid intima-media thickness than those without these receptors. The hypertension stage was associated with increased neutrophil but not monocyte adiponectin receptor density. Adiponectin is a peptide hormone produced by fat tissue and secreted into the bloodstream. High plasma adiponectin levels correlate with a lower risk of developing diabetes mellitus type 2, and low adiponectin levels are characteristic of obese patients. These crucial results show the interplay between arterial hypertension, fatty tissue, immunological mechanisms, and arterial damage [39]. A summary of the findings from pediatric studies on the immunological mechanisms of arterial damage in PH is presented in Table 1.
Table 1
Main results of pediatric studies on immunological mechanisms of arterial damage in pediatric hypertension
| Authors, publication year [reference] | Study design | Population (n) | Markers of inflammation | Markers of arterial damage | Main result |
|---|---|---|---|---|---|
| Gackowska et al., 2015 [38] | Cohort | PH – 57 CG – 19 | Leukocyte adiponectin receptor expression, MMP-9, TIMP-1, sCD14, IL-12p70, IL-1β, TNF-α, hsCRP | cIMT, local carotid artery stiffness | Positive correlation between cIMT and the presence of adiponectin receptor on leukocytes |
| Niemirska et al., 2016 [37] | Cohort | PH – 109 CG – 74 | MMP-9, TIMP-1, hsCRP | aPWV, AIx, cIMT, local carotid artery stiffness | Positive correlation between arterial stiffness and TIMP-1 |
| Gackowska et al., 2018 [35] | Cohort | PH – 34 CG – 35 | CD31, CCR7, CD28 receptor expression | aPWV, AIx, cIMT, local carotid artery stiffness | Positive correlation between arterial stiffness and CD4+CD31– cells (suggesting thymic dysfunction) |
| Gackowska et al., 2020 [36] | Cohort | PH – 33 CG – 35 | Number of Tregs and subsets of Tregs, hsCRP | aPWV, AIx, cIMT, local carotid artery stiffness | Positive correlation between arterial stiffness and decreased number of CD4+ Tregs, and elevated number of activated/memory CD4+ Tregs |
| Skrzypczyk et al., 2021 [12] | Cohort | PH – 119 CG – 45 | NLR, PLR, MPV | aPWV, AIx75HR, cIMT, local carotid artery stiffness by echo-tracking | Positive correlation between arterial stiffness and NLR |
[i] AIx – augmentation index, AIx75HR – augmentation index corrected for heart rate 75 beats per minute, aPWV – aortic pulse wave velocity, CCR – C-C chemokine receptor, CD – cluster of differentiation, CG – control group, cIMT – carotid intima-media thickness, hsCRP – high-sensitivity C-reactive protein, IL – interleukin, MMP – matrix metalloproteinase, MPV – mean platelet volume, NLR – neutrophil-to-lymphocyte ratio, PH – primary hypertension, PLR – platelet-to-lymphocyte ratio, TIMP – tissue inhibitor of metalloproteinases, s – soluble, TNF – tumor necrosis factor, Tregs – regulatory T-cells
Possible therapeutic approach to immunological disorders and arterial damage
Getting to know the mechanisms governing the processes of damaging vital structures in the course of AH not only allows us to understand the disease itself better but also gives us some idea of how to manage it. First of all, all studies analyzing the influence of the immune system show a significant role of inflammation in initiating and aggravating vascular damage, but also in the repair processes leading to arteriosclerosis and, furthermore, to atherosclerosis. Hence, it seems extremely beneficial to find therapeutic methods that will not only be effective in lowering blood pressure but will also inhibit inflammation or even reverse the effects of organ changes (HMOD). There are already studies, both experimental and on patients with PH, investigating the impact of known drug groups in inhibiting inflammation. For example, drugs from the group of RAAS inhibitors have been shown to be effective in reducing levels of inflammatory mediators such as CRP, IL-1β, IL-6, TNF-α, and ICAM-1, most of which have been proven to be associated with severe arterial damage. Moreover, they improve endothelial function by inhibiting the expression of endothelin-1 (ET-1) and increasing the bioavailability of nitric oxide (NO) [40].
Calcium channel blockers (CCBs) showed similar effectiveness to RAAS inhibitors. In addition to reducing the concentration of pro-inflammatory cytokines, the use of therapeutic doses of CCBs also reduced the levels of metalloproteinases – specifically MMP-2 and MMP-9, whose reduced activity inhibits the process of ECM degradation and vascular wall fibrosis [41].
Another group of anti-inflammatory drugs is mineralocorticoid receptor antagonists (MRAs). Aldosterone impairs the endothelium’s proper functioning by interfering with the proliferation and differentiation of endothelial cells. Its inhibition by MRAs resulted in inhibition of the NADPH oxidase pathway and increased NO secretion, as well as partially related improvement in endothelial function and inhibition of the vascular wall remodeling process [42].
It is worth mentioning that there are also studies examining the effectiveness of vitamin D in inhibiting inflammation. It has a proven anti-inflammatory and antimitotic effect, but it should be noted that so far, it has not been possible to confirm the effectiveness of its use in reducing inflammation in patients with PH, even in those diagnosed with a deficiency of < 20 ng/ml [43].
More is needed to confirm the importance of inflammation in the development of PH and HMOD. It is the identification of specific compounds related to the intensification of inflammation that creates new therapeutic possibilities. Knowing which specific pro-inflammatory cytokines and inflammatory mediators directly affect the process of vascular wall damage, we can look for drugs that act directly on these compounds. Monoclonal antibodies are used to inhibit the secretion of interleukins, mainly in autoimmune inflammatory diseases. The benefits of using PH-related interleukin inhibitors in animal models have already been demonstrated. For example, inhibiting IL-6 has been shown to be effective in reducing myocardial fibrosis and inflammation. On the other hand, studies on antibodies against the IL-6 receptor (IL-6R) did not show similar effectiveness but presented a wide range of side effects [44]. Of course, the research does not end with IL-6 only – the effectiveness of inhibitors of other inflammatory mediators is analyzed, although many studies have concluded that their use as antihypertensive drugs is inadvisable due to the predominance of their harmful effects on the patient’s system, e.g., neutropenia, leukopenia, infections and the development of the neoplastic process. However, this does not change the fact that the search for monoclonal antibodies directed against mediators associated with the development of HMOD is an interesting point of research [45] in the effective treatment not even of hypertension itself but its complications, which pose a number of threats to the patient’s life.
Not only pharmacotherapy of primary hypertension but also non-pharmacological treatment can modulate inflammation. In many studies, one of the main conclusions is the relationship between obesity and excess body weight with hypertension [46, 47]. The resultant dietary and exercise management as a weight loss method is one of the first therapeutic steps in lowering blood pressure, but it also has an anti-inflammatory effect. In addition, effective weight control goes hand in hand with the reduction of body weight by normalizing the lipid profile, which also inhibits the atherosclerotic process, one of the last stages of vascular remodeling. Another interesting mechanism is the observed effect of adipokines on vascular damage. This raises the question to what extent normalization of body weight will affect the secretion of adipokines and whether it will result in the inhibition of EVA [48].
Finally, a better understanding of the mechanisms of arterial damage related to disorders of the immune system creates not only therapeutic but also diagnostic possibilities. Especially at the molecular level, we can identify specific compounds, such as miRs, that may serve us in the future as EVA biomarkers, good prognostic markers of arterial stiffness, and anti-aging targets.
Summary
Primary hypertension is an increasingly common problem in the pediatric population. With every new study, we learn more and more about the consequences that may remain with the patient for life, but also about the mechanisms in which PH develops and leads to further changes. One of the most important concerns is the patient’s vessels. They can be damaged not only in a purely mechanical way by the hydrostatic pressure that blood exerts on vascular walls but also in a much less tangible way, which is the immunological disorders of a hypertensive patient. Extremely noteworthy is the phenomenon of inflammation, which can be both a cause and a consequence of hypertension. We can study its markers to try to assess how advanced the changes caused by being left untreated are, but also how effective the therapy is. The knowledge of PH-specific inflammatory mediators offers a chance to develop targeted drugs that will not only lower blood pressure itself but also impact the consequence, which is organ damage that shortens the patient’s life the most.