Introduction
Clonal micropropagation is a fast and convenient method of plant reproduction that enables the production of large amounts of planting material in a short period. This technique offers several advantages, including the ability to protect seedlings from viral and bacterial infections and the potential for year-round production of valuable plant material. Microclones occupy little space and, therefore, do not require a large area to grow like ordinary seedlings. In addition, it is possible to propagate plants of certain species and varieties that are difficult to reproduce using traditional methods. However, the effectiveness of micropropagation varies among different plant species, requiring specific approaches and adjustments to standard procedures. The success of micropropagation largely depends on the quality of the resulting seedlings and their ability to adapt from environmental conditions to the natural environment during acclimatization (Tytarenko et al., 2020). This process often leads to the loss of a large amount of plant material due to reduced resistance to infections, physiological limitations (such as poorly developed cuticles, excessive transpiration, and low photosynthetic efficiency), and inadequate stress tolerance compared to conventional plants (Shekhawat et al., 2021). Alleviating acclimatization stress for micropropagated plants is currently a key priority in plant biotechnology.
Paulownia, also known as the Princess tree (Paulownia tomentosa (Thunb.) Steud.), is a rapidly growing tree crop that has gained popularity in horticulture worldwide. Its easy cultivation, decorative value, and rapid growth make it highly sought after for large batches of paulownia seedlings. Traditional methods of reproduction through seeds or layering are less effective and do not guarantee healthy plants. Conversely, clonal micropropagation offers a more efficient approach to rapidly obtaining a large number of healthy paulownia seedlings (Crisan and Petrus-Vancea, 2016).
Common blackberry (Rubus fruticosus L.) is a traditional fruit and berry crop that is becoming increasingly popular in Ukrainian gardens. Thornless varieties are particularly favored due to their convenience for harvesting and excellent fruit quality. Although blackberries can be easily propagated using conventional vegetative methods, clonal micropropagation generates larger numbers of planting materials with desired characteristics (Fathy et al., 2018).
For both paulownia and common blackberry, clonal micropropagation not only proves to be effective and convenient but also offers economic benefits as a method of reproduction. The profitability of this process can be further enhanced by optimizing certain stages of micropropagation, which are known to result in the loss of plant material. One such critical stage is the acclimatization to ex vitro conditions. Considering that micro-propagated plants do not possess a well-established microbiota, and knowing the importance of the plant – microorganism interaction in nature, it is plausible to assume that inoculating microclones with beneficial bacteria during the acclimatization phase can increase the survival rate of plantlets in the soil and positively impact the biometric characteristics of the planting material.
Lactic acid bacteria have been recognized for their potential benefits to plants and are currently used as biocontrol agents against phytopathogens, plant growth promoters, biopesticides, remediation agents, and biofertilizers (Raman et al., 2022). These bacteria can be isolated from various plant parts and the rhizosphere, with the greatest diversity typically observed near the roots (Fhoula et al., 2013). Enterococcus bacteria are occasionally found in the plant rhizosphere, but their role in plant associations remains poorly studied. Mostly, enterococci are considered as part of the transient microbiota (Svec et al., 2012; Kumar et al., 2016). They exist as members of rhizosphere consortia (Strafella et al., 2021) and can play a significant role in plant resistance against biotic and abiotic stresses (Panwar et al., 2016).
Enterococci have been observed to produce phytohormones (Lee et al., 2015) and bacteriocins with different activity spectra (Okkers et al., 1999; H-Kittikun et al., 2015; Almeida-Santos et al., 2021). They can also synthesize biosurfactants, volatile compounds, and other substances that protect plants against phytopathogens, making them potential biocontrol agents (Kumar et al., 2016; David and Onifade, 2018; Maany et al., 2019; Chaurasia et al., 2021; Diaz et al., 2021).
The search for and identification of potentially beneficial plant-associated enterococci is an important area of research aimed at supporting sustainable agricultural practices (Cesa-Luna et al., 2019). The use of growth-promoting and protective microorganisms in plant cultivation can reduce the reliance on chemical fertilizers and toxic plant protection agents while increasing the yield of important agricultural crops. Enterococci with growth-promoting properties can also find applications in various plant-related fields, including clonal micropropagation.
Enterococcus italicus ONU547 was isolated from plant material at Odesa I. I. Mechnikov National University (Merlich et al., 2019). This strain has been extensively studied in the field of plant–microbe interactions. Certain characteristics of E. italicus ONU547, such as bacteriocin and organic acid production, inhibition of phytopathogenic bacteria growth, and the formation of biofilms on plant surfaces, suggest the potential of these bacteria for enhancing the growth and protection of R. fruticosus and P. tomentosa plants (Merlich et al., 2017a, 2017b, 2019).
Therefore, the aim of this study was to evaluate the growth-promoting and protective potential of E. italicus ONU547 for R. fruticosus and P. tomentosa plants, particularly during the effect of micropropagated plants to ex vitro conditions. To achieve this purpose, the antagonistic activity of the bacteria against phytopathogenic fungi was determined, a seed germination assay was conducted using Lepidium sativum L. as a model plant, and acclimatization experiments were performed on blackberry and paulownia microclones by inoculating the roots with the bacterial strain.
Materials and methods
Plant material
Young, nonlignified shoots of paulownia (P. tomentosa (Thunb.) Steud.) and blackberry (R. fruticosus L. var. Thornfree) were chosen as the initial plant materials for establishing in vitro cultures. The donor material was collected between 2018 and 2021 from plants grown in the open ground at the Botanical Garden of Odesa I.I. Mechnikov National University. Nodal segments containing apical or axillary buds were used as the initial explants.
After surface sterilization and cutting the shoots, nodal segments (n = 30 for each plant species) were cultured on Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) supplemented with 25 g/l sucrose and 1 mg/l of 6-benzylaminopurine (6-BAP). During the multiplication stage, the phytohormonal composition of the media was modified as follows: for blackberry plants, 1.5 mg/l 6-BAP and 0.5 mg/l naphthylacetic acid were used; for paulownia plants, 2 mg/l 6-BAP and 0.5 mg/l indolylacetic acid were used. The plant material (n = 60 for each plant species) was cultivated in an incubation room at 25°C, with a light intensity of 2500 lx, relative humidity of 56–70%, and an 8/16 h (night/day) photoperiod.
For the preacclimatization stage, 60 microclones (separately for blackberry and paulownia) were transferred to MS medium without phytohormones. During the acclimatization experiments, plants with a well-developed root system and similar heights ranging from 3.0 to 3.5 cm for R. fruticosus and 4.5 to 5.0 cm for P. tomentosa were used. At this stage, the microclones were divided into three groups, with each group consisting of 20 plants. Two groups were treated with different concentrations of bacteria, while the third group served as an uninoculated control. All experiments were repeated three times.
Microorganisms and incubation conditions
For this research, E. italicus ONU547, which is stored in the Ukrainian Collection of Microorganisms of D.K. Zabolotny Institute of Microbiology and Virology of the National Academy of Sciences of Ukraine (IMV NASU, Kyiv, Ukraine), was utilized. The bacteria were incubated in De Man, Rogosa, and Sharpe (MRS) liquid nutrient medium at 37°C for 24 h.
The tests strains of mycelial fungi, including Aspergillus niger F-16706, Paecilomyces variotii F-424, Cladosporium cladosporioides F-2235, Penicillium expansum F-575, Alternaria alternata F-16866, Fusarium oxysporum F-54201, were obtained from the collection of IMV NASU (Kyiv, Ukraine). The test strains of Rhizoctonia cerealis F-30 and Alternaria tenuissima F-24 were obtained from the collection of the Department of Microbiology, Virology, and Biotechnology of Odesa I.I. Mechnikov National University (Odesa, Ukraine). These selected species are commonly associated with plant diseases in field conditions and are also frequent contaminants in plant tissue culture (Omamor et al., 2007; El-Baky et al., 2021; Liu et al., 2021; Wang et al., 2021). The fungi were cultured on potato dextrose agar (PDA) at 28°C.
Antagonistic activity assay
The antagonistic activity of E. italicus ONU547 against mycelial fungi was determined using the agar block method. The bacteria were cultured overnight on MRS agar at 37°C. Subsequently, a block containing E. italicus ONU547 was cut from an agar plate and placed in a Petri dish on a PDA medium that had been freshly seeded with fungal spores. To obtain the fungal inoculum, A. niger F-16706, P. expansum F-575, and F. oxysporum F-54201 were incubated for 2 days until the sporulation stage, P. variotii F-424, C. cladosporioides F-2235, and R. cerealis F-30 were incubated for 3 days, and A. alternata F-16866 and A. tenuissima F-24 were incubated for 5 days. Fungal spore suspensions were prepared in a sterile physiological solution and inoculated on a PDA medium following the method described by Shobha and Kumudini (2012). After placing the bacterial blocks, the fungi were incubated at 28°C for 5 days, and the diameter of the growth inhibition zone was measured.
Study of the effect of E. italicus ONU547 on the growth of L. sativum L. plants
To determine the ability of E. italicus ONU547 to promote plant growth in vitro, a seed germination assay was conducted using watercress (L. sativum L.) as the model plant. The influence of the bacteria on the germination rate and biometric characteristics of watercress seedlings was assessed.
E. italicus ONU547 was cultured overnight in MRS liquid medium at 37°C with shaking at 180 rpm. The liquid culture was then centrifuged for 10 min at 3500 × g and washed twice with sterile water. Cell suspensions with concentrations of 106, 107, and 108 CFU/ml were prepared for inoculation.
Watercress seeds (n = 80) were surface sterilized in a 3.5% sodium hypochlorite solution for 20 min and washed three times with distilled water. The seeds were then placed in the bacterial suspensions for an hour, with 20 seeds in each group. Afterward, the seeds were transferred to sterile moist chambers for germination. The control group seeds were exposed only to distilled water.
Observations were performed over a period of 10 days. The average length of the shoots and roots of the watercress seedlings was measured, along with the germination rate and the number of roots per plant.
Acclimatization of micropropagated plants to ex vitro conditions
The acclimatization process consisted of two stages: preacclimatization and subsequent acclimatization. During the preacclimatization stage, holes were made in the lids of containers housing the microclones. The purpose of these holes was to establish gas exchange with the atmosphere to facilitate transpiration processes. The diameter of the holes gradually increased over the 5 days, starting with 0.5 cm for the first 3 days and then enlarging to 1 cm for the last 2 days). This contributed to the establishment of transpiration processes in drier air compared with high humidity in vitro (Leite et al., 2021).
After the preacclimatization stage, the microclones were carefully removed from the MS medium and washed with sterile water. The roots of the microclones were then inoculated with E. italicus ONU547 by immersing them in a suspension of the bacteria at different concentrations (106 and 107 CFU/ml) for 1 h. In contrast, the roots of plants in the control group were treated with distilled water. The microclones were subsequently planted in autoclaved soil mixed with 10% agroperlite and placed in an acclimatization room with controlled environmental conditions. The acclimatization room maintained a temperature range of 22–24°C, with a light intensity of 2500 lx, relative humidity of 56–70%, and an 8/16 h (night/day) photoperiod.
During the first 2 weeks, the microclones were irrigated with sterile tap water twice a week. Afterward, irrigation was continued using nonsterile tap water at the same frequency.
Observations were conducted over a period of 30 days after planting. On the 7th, 14th, and 30th days of cultivation, the survival rate of the microclones in the soil was measured, along with several biometric characteristics including average shoot length, number of nodes, and leaf area.
The survival rate was determined as the percentage of viable plants among all the plants in the experimental group. The average shoot length and number of nodes were manually measured. The ImageJ software, as described by Cosmulescu et al. (2020), was utilized to measure the average leaf area.
Statistical analysis
All experiments were performed in triplicate, and the data are presented as the mean values ± standard deviations. The plant material was randomly chosen and distributed. Statistical analysis was carried out using a one-way analysis of variance, followed by Duncan’s multiple range test (DMRT) utilizing the Statistical Package for the Social Sciences software, version 29. Significance was determined at P ≤ 0.05.
Results
Antagonistic activity of E. italicus ONU547 against phytopathogenic fungi
E. italicus ONU547 showed antagonistic activity against seven strains of mycelial fungi (Table 1). The largest zones of growth inhibition were observed when exposed to A. niger (inhibition zone diameter: 19.3 ± 1.2 mm), C. cladosporioides (18.7 ± 1.2 mm), and A. alternata (16.7 ± 1.2 mm). Antagonistic activities were also identified against A. tenuissima, R. cerealis, and P. variotii, with average growth inhibition zones ranging from 14.0 to 14.7 mm. Furthermore, E. italicus ONU547 exhibited antagonism against P. expansum, characterized by delayed sporulation rather than the absence of mycelial growth. However, no antagonistic activity against F. oxysporum was observed.
Table 1
Antagonistic activity of E. italicus ONU547 against mycelial fungi
| Fungi strains | Average growth inhibition zone diameter [mm] |
|---|---|
| A. tenuissima F-24 | 14.7 ± 1.2 |
| R. cerealis F-30 | 14.0 ± 0.0 |
| F. oxysporum F-54201 | 0.0 ± 0.0 |
| C. cladosporioides F-2235 | 18.7 ± 1.2 |
| P. variotii F-424 | 14.7 ± 1.2 |
| P. expansum F-575 | 14.0 ± 0.0 * |
| A. alternata F-16866 | 16.7 ± 1.2 |
| A. niger F-16706 | 19.3 ± 1.2 |
Effects of E. italicus ONU547 on germination and seedlings growth of L. sativum L.
To determine the growth-promoting potential and potential phytotoxic effect of E. italicus ONU547, a screening assay was conducted using watercress as a model plant. The assay evaluated the effects on seed germination rate on the 2nd and 3rd days, as well as the average number of roots per seedling and the average length of shoots and roots on the 7th and 9th days of cultivation.
The results showed that the effects of different concentrations of E. italicus ONU547 on the mentioned characteristics varied compared to the control and between each other (Fig. 1).
Fig. 1
The effect of E. italicus ONU547 on the germination rate of watercress seeds on the 2nd and 3rd days of cultivation; control seeds (n = 20) received no treatment while treated seeds (n = 20 for each concentration) were inoculated with bacteria; means followed by a common letter are not significantly different by the DMRT at P ≤ 0.05
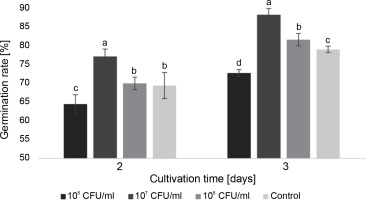
At a concentration of 107 CFU/ml, the bacteria had a positive impact on germination, increasing the germination rate by 7.8 and 9.3% on the 2nd and 3rd day, respectively. Bacteria at a concentration of 106 CFU/ml did not cause statistically significant changes compared to the control on the 2nd day, but they increased the germination rate by 2.5% on the following day. On the other hand, at a concentration of 108 CFU/ml, the bacteria exhibited a certain inhibitory effect on seed germination, with rates 5.0% lower than the control on the 2nd day and 6.3% lower on the 3rd day.
Overall, on the 3rd day of the experiment, a significant difference in germination rate was observed among all experimental groups, with the highest proportion of germinated seeds observed after inoculation with bacteria at a concentration of 107 CFU/ml (Fig. 1).
Regarding the average number of roots on the 7th and 9th days of cultivation, a statistically significant difference was observed between all experimental groups and the control (Fig. 2).
Fig. 2
The average number of roots in watercress seedlings on the 7th and 9th days after inoculation with E. italicus ONU547; control seeds (n = 20) received no treatment while treated seeds (n = 20 for each concentration) were inoculated with bacteria; means followed by a common letter are not significantly different by the DMRT at P ≤ 0.05
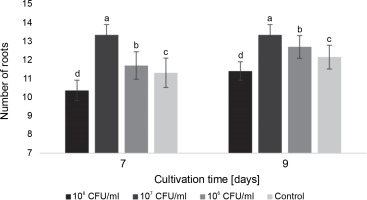
On the 7th day, the seedlings exposed to 107 CFU/ml of E. italicus displayed the highest average number of roots (13.4 ± 0.6 roots). Although the average number remained the same on the 9th day for these seedlings and increased by 0.9 roots in the control, the plants inoculated with 107 CFU/ml of bacteria still had the highest number of roots among all experimental groups. Conversely, seedlings exposed to 108 CFU/ml of E. italicus showed inhibition of root formation on the 7th and 9th days, while those inoculated with 106 CFU/ml showed a minimal level of positive influence (Fig. 2).
Significant differences were observed among all experimental groups in terms of the average length of roots and shoots on the 7th and 9th day (Fig. 3).
Fig. 3
The average length of roots and shoots of watercress seedlings on the 7th day after inoculation with E. italicus ONU547; control seeds (n = 20) received no treatment while treated seeds (n = 20 for each concentration) were inoculated with bacteria; means followed by a common letter are not significantly different by the DMRT at P ≤ 0.05
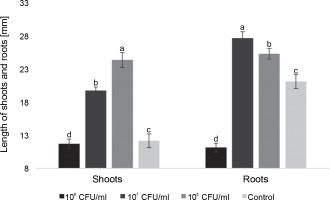
On the 7th day, seedlings inoculated with 106 CFU/ml of E. italicus exhibited the longest average shoot length, reaching 24.4 ± 1.1 mm. Those treated with 107 CFU/ml had an average shoot length of 19.7 ± 0.6 mm, while the group with 108 CFU/ml showed shoot lengths close to the control values (11.7 ± 0.7 mm). The average length of roots was highest in seedlings exposed to 107 CFU/ml of E. italicus (27.7 ± 1.0 mm), followed by those Inoculated with 106 CFU/ml (25.3 ± 0.8 mm). The control group exhibited an average root length of 21.1 ± 1.1 mm, while the group treated with 108 CFU/ml of E. italicus ONU547 had an average root length of 11.1 ± 0.7 mm (Fig. 3).
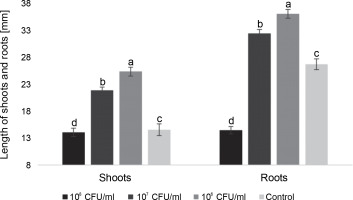
On the 9th day, the trends for most characteristics remained consistent. The highest average shoot length was observed in seedlings treated with 106 CFU/ml and 107 CFU/ml of E. italicus (21.8 ± 0.6 and 25.3 ± 0.8 mm, respectively). The group with 108 CFU/ml showed shoot lengths 0.5 mm shorter than the control, while the root length in this group was almost two times shorter (14.4 ± 0.4 and 26.6 ± 1.0 mm). Seedlings treated with 107 CFU/ml of E. italicus on the 9th day exhibited an average root length of 32.3 ± 0.7 mm, while the highest root length was observed in plants inoculated with 106 CFU/ml of bacteria (35.9 ± 0.8 mm) (Fig. 4).
Fig. 4
The average length of roots and shoots of watercress sprouts on the 9th day after inoculation with E. italicus ONU547; control seeds (n = 20) received no treatment while treated seeds (n = 20 for each concentration) were inoculated with bacteria; means followed by a common letter are not significantly different by the DMRT at P ≤ 0.05
In conclusion, the impact of E. italicus ONU547 on plants was found to depend on the bacterial inoculum concentration. In this study, a concentration of 108 CFU/ml exhibited phytotoxic effects, while concentrations of 106 CFU/ml and 107 CFU/ml demonstrated plant growth-promoting properties.
Effects of E. italicus on acclimatization of P. tomentosa and R. fruticosus microclones to ex vitro conditions
During the acclimatization process of micropropagated plants to nonsterile environmental conditions, E. italicus ONU547 demonstrated a favorable impact on the biometric characteristics of the seedlings, as well as on their survival rate in the soil, when compared to the control group. This effect was observed in both experimental plant species, namely blackberry and paulownia.
Survival rate
The survival calculations revealed that on the 30th day, the highest average number of viable R. fruticosus plants was observed in the group of microclones inoculated with 107 CFU/ml of E. italicus ONU547 (67.4 ± 1.2%). This resulted in a 15.9% increase in survival rate compared to the control group. In the group of microclones exposed to 106 CFU/ml of bacteria, the survival rate increased by 12.2% on the final day of observation (Table 2).
Table 2
The average survival rate of Rubus fruticosus and Paulownia tomentosa microclones in the soil after inoculation with E. italicus ONU547
Similarly, the survival rate of paulownia plants showed the greatest improvement when inoculated with 107 CFU/ml of bacteria. On the 30th day, it reached 73.7 ± 0.5%, which was 6.7% higher than the control group. With the inoculation of 106 CFU/ml of E. italicus, the survival rate reached 72.6 ± 0.5%, representing a 5.6% increase compared to the control (Table 2).
Furthermore, the study identified differences in the effects of different bacterial concentrations on the plants. For blackberry microclones, it was observed that on the 7th day of acclimatization, bacteria concentrations of 106 and 107 CFU/ml had a similar effect. On the 14th day, the concentration of 107 CFU/ml of E. italicus demonstrated greater effectiveness than 106 CFU/ml, but by the 30th day, both experimental concentrations showed a comparable impact. During the acclimatization of paulownia microclones, only E. italicus at a concentration of 107 CFU/ml exhibited a statistically significant effect on the survival rate on the 7th day. However, on the 14th and 30th days, the effects of bacteria at both concentrations were statistically similar (Table 2).
Therefore, the utilization of E. italicus ONU547 at concentrations of 106 and 107 CFU/ml led to an increase in the survival rate of R. fruticosus and P. tomentosa microclones compared to the control group.
Plant length
E. italicus ONU547 also had an impact on plant growth. The increase in the average length of blackberry and paulownia microclones during the acclimatization period from the 1st to the 30th day is illustrated in Figures 5 and 6.
Figure 5 demonstrates that the average length of blackberry plants naturally increased over time, with the greatest growth observed in microclones treated with 107 CFU/ml of bacteria. On the 30th day, their length was, on average, 2.3 cm longer than that of the control plants. From the 7th day until the end of the observation period, the concentration of 107 CFU/ml exhibited a distinct effect on the average length of plants compared to both the control and the concentration of 106 CFU/ml. Although 106 CFU/ml of E. italicus also displayed an effect, the acceleration of growth was observed with a delay compared to the 107 CFU/ml concentration. A significant difference from the control was observed only on the 30th day when the plants in this group were longer than the control plants by an average of 0.9 cm. However, no discernible difference in the effect of this experimental group compared to the control was detected from the first to the 14th day of acclimatization.
Fig. 5
The average length of the Rubus fruticosus microclones after inoculation with E. italicus ONU547; control plants (n = 20) received no treatment while treated plants (n = 20 for each concentration) were inoculated with bacteria; means followed by a common letter are not significantly different by the DMRT at P ≤ 0.05
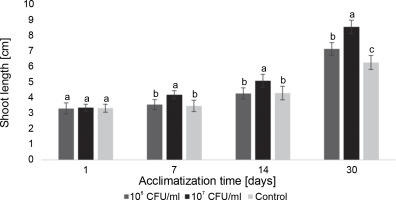
Fig. 6
The average length of the Paulownia tomentosa microclones after inoculation with E. italicus ONU547; control plants (n = 20) received no treatment while treated plants (n = 20 for each concentration) were inoculated with bacteria; means followed by a common letter are not significantly different by the DMRT at P ≤ 0.05
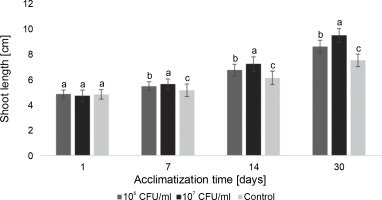
During the acclimatization of paulownia plants, differences between the control group and inoculated microclones were also observed (Fig. 6). Plants exposed to E. italicus ONU547 at both concentrations exhibited greater shoot growth throughout the entire observation period. On the 30th day, the average length of paulownia plants inoculated with 106 and 107 CFU/ml of bacteria was 9.5 ± 0.5 and 8.7 ± 0.5 cm, respectively, while control plants had an average height of 7.6 ± 0.5 cm. Differences in the effect of the experimental concentrations of E. italicus compared to the control and among themselves were observed from the 7th day until the end of the experiment.
Node number
Figure 7A depicts the average number of nodes in blackberry plants from the day of planting to the 30th day of acclimatization. The results obtained indicate a correlation between the number of nodes and the length of the plants, with a steady increase over time. Additionally, the difference between the inoculated plants and the control group also increased throughout the 30-day observation period. Notably, from the 7th day until the end of the experiment, a discernible difference in the effect of the experimental concentrations compared to the control group and among themselves was observed. The control plants consistently exhibited the lowest mean values over the course of the 30 days. The use of 106 CFU/ml of E. italicus resulted in intermediate results, with 7.5 ± 0.5 nodes observed on the 30th day. The highest number of nodes (an average of 8.8 ± 0.4 nodes/plant) was found in the group of plants inoculated with 107 CFU/ml of bacteria.
Fig. 7
The average number of nodes in the microclones after inoculation with E. italicus ONU547: A – Rubus fruticosus and B – Paulownia tomentosa ; control plants (n = 20) received no treatment while treated plants (n = 20 for each concentration) were inoculated with bacteria; means followed by a common letter are not significantly different by the DMRT at P ≤ 0.05
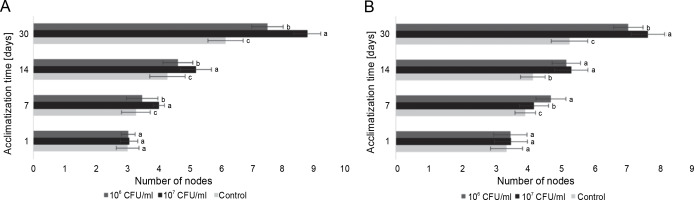
Similarly, for paulownia microclones, E. italicus ONU547 also increased the number of nodes in plants starting from the 7th day of acclimatization (Fig. 7B). However, in this case, both concentrations used in the experiment exhibited varying effects. On the 7th day of observation, the highest average number of nodes was observed in plants inoculated with 106 CFU/ml of E. italicus. On the 14th day, the effects of both concentrations were determined to be the same, and on the last day, the highest average number of nodes was found in microclones inoculated with 107 CFU/ml of bacteria (7.7 ± 0.5 nodes). Therefore, E. italicus ONU547 had a positive effect on the number of nodes in paulownia plants at both experimental concentrations.
Leaf area
Differences between the experimental and control plants were also noticeable in terms of the average leaf area in both blackberry and paulownia plants (Fig. 8A and Fig. 8B).
Fig. 8
Microclones on the 14th day of acclimatization with E. italicus ONU547: A – Rubus fruticosus (1–2: control, 3–4: inoculated with bacteria); B – Paulownia tomentosa (1–6: control, 7–12: inoculated with bacteria); control plants (n = 20) received no treatment while treated plants (n = 20) were inoculated with 107 CFU/ml of bacteria

Measurements of the average leaf area in blackberry plants during the acclimatization experiment differed from those of the control group. However, on the last day of observation, both experimental concentrations of bacteria showed similar results (Fig. 9). On the 7th day of acclimatization, a statistically significant difference in the average leaf area of blackberry plants compared to the control group was observed only in the microclones inoculated with 107 CFU/ml of E. italicus. On the 14th day, both experimental concentrations exhibited differences from the control group as well as from each other. On the 30th day, the average leaf area of the experimental plants was equal to the control and was 0.6 cm2 larger.
Fig. 9
The average leaf area of Rubus fruticosus microclones after inoculation with E. italicus ONU547; control plants (n = 20) received no treatment while treated plants (n = 20 for each concentration) were inoculated with bacteria; means followed by a common letter are not significantly different by the DMRT at P ≤ 0.05
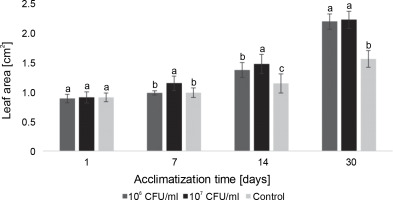
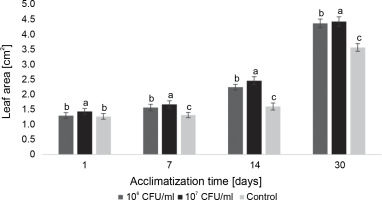
In the case of paulownia plants, bacteria at both experimental concentrations contributed to an increase in the average leaf area, with the highest average leaf area recorded in microclones inoculated with 107 CFU/ml of E. italicus throughout the entire observation period (Fig. 10). The effects of the studied bacterial concentrations varied over the course of the experiment. Although the difference in absolute values between leaf areas in plants exposed to 107 and 106 CFU/ml of bacteria was small, the statistical analysis revealed a significant difference between them. Therefore, the best effect on the average leaf area of paulownia was demonstrated by E. italicus at a concentration of 107 CFU/ml, which was 0.8 cm2 larger than the control on the 30th day of acclimatization.
Fig. 10
The average leaf area of Paulownia tomentosa microclones after inoculation with E. italicus ONU547; control plants (n = 20) received no treatment while treated plants (n = 20 for each concentration) were inoculated with bacteria; means followed by a common letter are not significantly different by the DMRT at P ≤ 0.05
Hence, the inoculation of R. fruticosus and P. tomentosa microclones with E. italicus ONU547 at concentrations of 106 and 107 CFU/ml exhibited a beneficial impact on various plant parameters, including the survival rate, average plant length, node count, and leaf area, when compared to the control group. Notably, the application of bacteria at a concentration of 107 CFU/ml yielded the most favorable results in terms of plant acclimatization to ex vitro conditions.
Discussion
This study represents the first comprehensive exploration of the potential application of E. italicus ONU547 as a plant growth promoter and beneficial microorganism for acclimatization. The antagonistic activity of the investigated bacteria against mycelial fungi, including A. niger, C. cladosporioides, A. alternata, A. tenuissima, R. cerealis, P. expansum, and P. variotii, was successfully established. These findings align with previous research conducted by other scholars, who have also detected antagonistic properties in various strains of enterococci (Kumar et al., 2016; Maany et al., 2019; Diaz et al., 2021). Notably, the authors did not find any prior reports on the antagonism of Enterococcus spp. against P. variotii, R. cerealis, A. tenuissima, and C. cladosporioides. However, the inhibitory effects of enterococci on other tested fungi species, such as A. niger, A. alternata, and P. expansum, were already documented in existing literature (Fhoula et al., 2013; Ben Braïek et al., 2018; David and Onifade, 2018; Zabouri et al., 2021), corroborating the findings of our experiments, which showcased similar antagonistic properties.
It is worth noting that the studied bacteria did not exhibit any antagonistic activities against F. oxysporum. This sets E. italicus ONU547 apart from related strains that have demonstrated inhibitory effects on the growth of Fusarium spp. in other studies (Ben Braïek et al., 2018; Kalantari et al., 2019; Houicher et al., 2021). Additionally, although the bacteria did not impede the growth of P. expansum mycelia, they did delay sporulation during cocultivation. This suppression of sporulation has the potential to decelerate the progression of fungal diseases in plants by influencing the subsequent dissemination of spores—a critical factor in disease spread (Dahlberg and Van Etten, 1982).
In previous in vivo studies (Merlich et al., 2017a), it was observed that E. italicus ONU547 exhibited inhibitory effects against Rhizobium radiobacter C58 and Erwinia carotovora ZM1 on carrot explants, resulting in growth reductions of 33.3 and 24.8%, respectively. These findings highlight the antagonistic potential of the microorganisms within the studied strain against both fungal and bacterial phytopathogens.
Furthermore, this study revealed the growth-promoting properties of E. italicus ONU547 for microclones during the transition from in vitro conditions to a nonsterile environment. Moreover, our experiments demonstrated the positive effects of inoculating with cell suspensions of the microorganisms rather than their purified metabolites, as often reported in the existing literature (Lee et al., 2015; Pellegrini et al., 2020; Chaurasia et al., 2022; Msimbira et al., 2022). This indicates that for the potential development of biological preparations based on E. italicus ONU547, there is an opportunity to save time by bypassing the isolation and purification of specific synthesized compounds and simply utilizing the cell mass. Such an approach holds promise for the creation of more cost-effective preparations.
The concentration of bacteria during inoculation has been determined to be a crucial factor in its impact on plants. For example, when watercress seeds were inoculated in vitro, a concentration of 108 CFU/ml was found to be phytotoxic, inhibiting germination and plant growth. On the other hand, concentrations of 106 CFU/ml and 107 CFU/ml exhibited growth-promoting properties. Based on measurements of root and shoot length on the 9th day of observation, a concentration of 106 CFU/ml was deemed more effective. However, the highest germination rate and the greatest number of roots were observed in seedlings inoculated with 107 CFU/ml of E. italicus.
In the acclimatization experiments, it was found that inoculating plants with bacteria at a concentration of 107 CFU/ml yielded the most significant outcomes in terms of all evaluated characteristics. A statistically significant difference from the control group was observed from the 7th day of cultivation and remained evident throughout the observation period. Bacteria at a concentration of 106 CFU/ml also demonstrated a positive effect, although it generally required more time to manifest and exhibited a lesser extent of the impact.
In other studies investigating the effects of enterococci on plant growth, different concentrations of bacteria, inoculation procedures, and plant species were employed, resulting in varying observed influences. For example, Chaurasia et al. (2022) inoculated soil with Enterococcus spp. at a concentration of 8 × 105 CFU/kg, leading to maize seed germination occurring three days faster than in the control group and a 53.7% increase in plant height, while no significant difference in the number of leaves was observed. In Kumar et al.’s (2016) experiments, Vigna mungo seeds were inoculated with a bacterial suspension (108 CFU/ml) mixed with 1% carboxymethyl cellulose, resulting in a 16.7% increase in germination rate, 23.7% increase in shoot length, and 27.3% increase in root length. Lee et al. (2012) found that bacterial inoculation significantly enhanced the shoot length of rice plants but did not yield positive effects on any of the analyzed biometric characteristics of cucumber plants. Simultaneously, the cell-free extract did increase the shoot length of plants by 40%. The exact concentration of the bacterial inoculum and the specific inoculation procedure was not specified in that research.
In the present study, the inoculation of L. sativum L. seeds with E. italicus ONU547 at a concentration of 107 CFU/ml through a 1 h soaking process resulted in a 9.3% increase in seed germination, 50.3% increase in in vitro shoot length, and 21.4% increase in root length compared to the control group. Additionally, for blackberry microclones during ex vitro acclimatization, the shoot length, node number, and leaf area exhibited increases of 36.5, 41.9, and 37.5% respectively. Similarly, for paulownia plants, the shoot length, node number, and leaf area increased by 25.0, 45.2, and 22.2%, respectively. A comparison of the aforementioned results suggests that the interactions between enterococci and plants can be species-specific and may depend on the inoculation procedure and inoculum concentration. Therefore, further extensive research is warranted to explore this topic.
The observed effects of E. italicus ONU547 on seed germination and the growth of micropropagated plants during the acclimatization stage can be preliminarily explained by several factors. It has been established that E. italicus ONU547 is a producer of organic acids, predominantly acetic acid (Merlich et al., 2017a), which may contribute to its antagonistic activity against phytopathogens. Additionally, E. italicus ONU547 has been found to produce bacteriocin, which exhibits increased activity after heating and demonstrates antagonistic effects against phytopathogenic bacteria (Merlich et al., 2019).
Previous studies have indicated that E. italicus ONU547 can form biofilms on the surface of watercress roots, particularly when in consortia with other lactic acid bacteria. This adhesive property plays a significant role in effective plant protection and facilitates the better penetration of bacterial metabolites into the intercellular space (Merlich et al., 2017b).
The growth-promoting properties observed in this study may be attributed to the potential synthesis of phytohormones, as similar traits have been reported for enterococci (Lee et al., 2015). Additionally, the studied strain could potentially have a positive impact on plants through nitrogen fixation and phosphate solubilization, as demonstrated by other research on enterococci (Mello et al., 2020). Further investigations will be necessary to verify these hypotheses specifically for E. italicus ONU547.
Conclusion
E. italicus ONU547 demonstrated antagonistic activity against various phytopathogenic fungi, including C. cladosporioides, A. alternata, A. tenuissima, A. niger, R. cerealis, P. variotii, and P. expansum. Furthermore, the bacteria exhibited plant growth-promoting properties in the germination assay of L. sativum L. seeds, leading to increased average root and shoot length, the average number of roots, and germination rate compared to the control group. Additionally, positive effects were observed in the ex vitro acclimatization of micropropagated P. tomentosa Steud. and R. fruticosus L. plants, including an increase in average plant length, number of nodes, leaf area, and survival rate in the soil. The optimal inoculum concentration for effective acclimatization of microclones was determined to be 107 CFU/ml.
Given the outcomes of these experiments and the various potential mechanisms by which E. italicus ONU547 impacts plant tissues, further research on these bacteria is recommended. They show promise for inoculating micropropagated plants during the acclimatization stage to ex vitro conditions.










