Summary
The impact of direct stenting on small vessel coronary culprit lesions in patients with ST-segment elevation myocardial infarction has not been investigated yet. The present study clearly emphasizes that direct stenting in selected lesions appears to be a safe and successful procedure, providing lower stent thrombosis and procedural complication rates. The procedural and radiation exposure time, and contrast load were also lower in the DS group.
Introduction
The primary therapeutic strategy in patients with acute ST-elevation myocardial infarction (STEMI) is percutaneous coronary intervention (PCI) with stent implantation [1]. The conventional stenting (CS) technique requires routine pre-dilatation with the balloon catheter to ensure easy passage of the stent and to increase the full expansion of stent. With the innovation in stent and delivery system design, direct stenting without balloon pre-dilatation has become a feasible strategy in many catheterization laboratories [2]. Direct stenting (DS) without lesion pre-dilatation is employed in 30–40% of PCIs and has been compared to stenting with pre-dilatation in observational studies as well as randomized trials. DS without balloon pre-dilatation is thought to provide beneficial effects by reducing distal embolization and thereby microvascular obstruction [3]. Prospective studies as well as meta-analyses demonstrate 22–50% reductions in restenosis, target lesion revascularization (TLR), myocardial infarction (MI) and death associated with DS compared to CS [4–6]. Pre-dilation may induce intimal dissection necessitating multiple or altogether longer stents, increasing the risk of restenosis [7]. DS also offers significant reductions in procedure time, radiation exposure, contrast administration, and adjunctive material, use and also cost reduction was achieved by direct stenting [8]. However, a number of disadvantages have been suggested for DS, including failure to cross the lesion, incomplete stent deployment, an increase in guide trauma, undersizing the stent, and poor visualization, which may result in errors in stent positioning [9]. It also might increase procedural risks and may lead to suboptimal clinical results.
Aim
There are no previous studies comparing DS with a conventional approach for small vessel coronary artery disease (CAD) for clinical outcomes of patients with ST-elevation myocardial infarction (STEMI). The aim of the study was to assess in-hospital and long-term clinical outcomes of STEMI patients with small vessel CAD treated with direct stenting as compared with those treated with stenting after pre-dilatation.
Material and methods
Study design and population
This was a retrospective, observational, single-center study. The current study included 616 consecutive patients undergoing a primary PCI with stenting for small vessels (≤ 2.75 mm) in Sakarya University Education and Research Hospital, Sakarya, Turkey from January 2013 to January 2017. The patients were divided into two groups, treated with DS (n = 202, 32.8%) or CS (n = 414, 67.2%). Small vessel CAD was considered a need for implantation of stents ≤ 2.75 mm (diameter of the reference vessel and diameter of the implanted stent: ≤ 2.75 mm). All patients > 18 years of age presenting with STEMI within 12 h of symptom onset or between 12 and 24 h if they had persistent symptoms with evidence of ongoing ischemia were included in the study. DS was the primary modality of treatment in all patients wherever possible. All patients where DS was done formed the direct stenting group (DS group), and the rest were included in the CS group. Patients in the CS group underwent balloon pre-dilatation prior to stenting. The exclusion criteria were a concomitant large diameter PCI in the same coronary artery, left main coronary artery lesions, contraindications to inhibit platelet function with aspirin and clopidogrel, cardiogenic shock, a PCI consisting of in-stent restenosis (ISR) for the culprit lesion, life expectancy < 12 months and pregnancy. Lesions that were heavily calcified or associated with excessive proximal tortuosity were also excluded. An additional exclusion criterion was a lack of relevant patient or procedural related data.
This study complied with the Declaration of Helsinki, and it was approved by the independent medical ethics committee of Sakarya University Education and Research Hospital.
Study protocol
During primary PCI, those patients who had thrombolysis in myocardial infarction (TIMI) flow of ≥ 1 at initial injection or after wire placement underwent DS. Stenting was performed after pre-dilatation in patients whose vessel was still completely occluded after insertion of the wire. Coronary stenting was considered angiographically successful if residual stenosis of < 30% and coronary thrombolysis in myocardial infarction grade flow 3 were obtained at the end of the procedure. During the procedure, an intra-arterial bolus of unfractionated heparin was given at a dose of 80 U/kg. After the intervention, all patients received aspirin indefinitely, clopidogrel, prasugrel or ticagrelor for at least 12 months and other cardiac medications according to ACC/AHA guidelines [10]. Angiographic findings such as vessel dimensions, pre- and post-procedural stenoses and lesion length were determined by visual estimation using the guiding catheter as a reference object for calibration. The angiographic characteristics were also further analyzed by an independent interventional cardiologist not involved in the procedure and checked for inter-observer agreement.
Data collection
Baseline demographics, clinical characteristics and procedural data were collected retrospectively. Thrombus burden, calcification status and postprocedural TIMI flow grade were evaluated by two experienced interventional cardiologists. The contrast volume, procedural time and fluoroscopy time data were obtained from the records of the coronary angiography laboratory. The in-hospital and 2-year follow-up information on clinical outcomes (e.g. in- hospital death, recurrent MIs, TLR, target vessel revascularization (TVR) and definite ST) were collected from electronic medical records, a registry database or phone calls, which asked about relevant end-point clinical events. Routine or control angiography during the follow-up without a clinical indication was not undertaken. However, event-driven coronary angiographies after the initial PCI were performed within the 2-year follow-up period.
Study endpoints and definitions
The primary end-point of the study was the composite of major adverse cardiac events (MACEs), which were defined as TLR, TVR, MI or definite ST during the follow-up period. The secondary end-points included in-hospital TLR and in-hospital death. TVR was defined as any clinically driven PCI or bypass grafting of the target vessel. TLR was defined as any clinically driven repeat PCI or bypass grafting of the treated lesion, including the placement of an in-stent or in-segment 5 mm proximal or distal to the initial stent edges. An MI was defined according to current guidelines [11]. Definite ST was defined based on the criteria of the Academic Research Consortium [12]. Total ischemic time was defined as the time from the onset of chest pain to the first balloon inflation during primary PCI. Angiographic thrombus burden was graded using TIMI thrombus classification, and it was classified as low thrombus burden (grades 1, 2 and 3) and high thrombus burden (grades 4 and 5) [13]. In this study, no-reflow and edge dissection were defined as procedural complications.
Statistical analysis
For the statistical analysis, SPSS version 16.0 for Windows (SPSS Inc., Chicago, IL) was used. Continuous data were expressed as mean ± standard deviation, and the categorical data were expressed as percentages. The normal distribution of the data was assessed by the Kolmogorov-Smirnov test. Comparisons between groups were performed using the χ2 or Fisher’s exact test for qualitative variables, as appropriate. The independent t-test was used for normally distributed continuous variables, and the Mann-Whitney U test was conducted for non-normally distributed continuous variables, as appropriate. The baseline characteristics and clinical outcomes of the patients treated with direct stenting versus those treated with pre-dilatation prior to stenting were compared. Kaplan-Meier analysis was used to calculate the time to the clinical end-point, and the log-rank test was applied to compare between-group differences. To determine the impact of the DS strategy on 2-year ST, multivariate logistic analysis was performed. Clinical, procedural and angiographic criteria were entered into a univariate model with 2-year ST as the dependent variable. Variables reaching significance, or borderline significance, on univariate analysis (p ≤ 0.1) were subsequently incorporated into a multivariate model. Independent variables are presented as odds ratios with 95% confidence intervals. The Cox model included age, gender, multivessel disease, stent length, stent diameter, target vessel, lesion location, complication, postdilatation, prior PCI, prior MI, prior CABG, diabetes mellitus (DM), hypertension (HT), hyperlipidemia, smoking, renal failure, thrombus burden, left ventricular ejection fraction (LVEF), and DS. For multivariate analysis, the following covariates were considered for entry into the multivariate models (only those with p-values < 0.10 were retained via stepwise regression): DS, gender, prior PCI, prior MI, and stent diameter. A p-value < 0.05 was considered statistically significant in all tests.
Results
Characteristics of patients
The baseline demographics and baseline clinical characteristics of groups are shown in Table I. The two groups were comparable in terms of demographics and clinical characteristics and all values were similar between the two groups (p > 0.05). However, significantly lower baseline LVEF (p = 0.009) and higher age (p = 0.019) were found in the CS group. The total ischemic time was found similar in both groups.
Table I
Baseline demographics and clinical characteristics
Characteristics of the lesions, and PCI procedures
The lesional and procedural characteristics are summarized in Table II. There was no difference in terms of culprit artery and lesion location between groups. In both groups, the lesions were located mostly in the middle part of the vessel and the culprit artery was mostly the right coronary artery (RCA). Moreover, the stents were significantly longer (p = 0.001) and stent diameter was lower (p = 0.050) in the CS group. The incidence of procedural complications were significantly higher in the CS group as compared with the DS group (p = 0.01). The DS compared to CS resulted in greater rates of postprocedural TIMI grade 3 flow (p = 0.048) and lower risk of edge dissection (p = 0.038). However, there was no difference between the two groups with regards to thrombus burden, calcification, or rate of aspiration thrombectomy. DS when compared with the CS technique significantly decreased both procedure time (43.5 ±12.6 min vs. 47.7 ±15.2 min, p < 0.001) and fluoroscopy time (9.7 ±4.4 min vs. 11.7 ±5.7 min, p < 0.001). Moreover, contrast volume used in the DS group was significantly lower than the CS group (128.8 ±52.6 ml vs. 151.3 ±83.7 ml, p < 0.001).
Table II
Lesions and procedural characteristics
Clinical outcomes
At the 2-year follow-up, 52 (25.7%) patients in the DS group and 98 (23.7%) patients in the CS group needed to undergo angiographic evaluations. The outcomes of the patients during the follow-up period are summarized in Table III. At the 2-year follow-up, the primary composite end-point, MACEs, occurred in 9.2.% of the DS group and 12.3% of the CS group (p > 0.05). There was also no significant difference in TVR and MI rates between the two groups after 2 years (p > 0.05). Moreover, there was no significant difference between groups in TLR rates during hospitalization (p > 0.05). Although TLR and in-hospital death tended to be lower in the DS group, the differences between groups were not significant (p = 0.07 and p = 0.08, respectively). However, the ST rate was significantly higher in the CS group as compared with the DS group (p = 0.04).
Table III
Clinical outcomes at 2 years and in-hospital
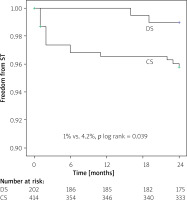
Kaplan-Meier survival analysis revealed no difference in event-free MACE ratio between the patients treated with DS and CS (9.2% vs. 12.3%; p log rank > 0.05) (Figure 1). The time-to-event curves reflected a higher incidence of ST in the CS group for small-vessel culprit lesions (4.2% vs. 1.0%; p log rank = 0.039) (Figure 2).
Figure 1
Kaplan-Meier curve estimate of freedom from major adverse cardiac events (MACE) at 24-month follow-up
DS – direct stenting, CS – conventional stenting.
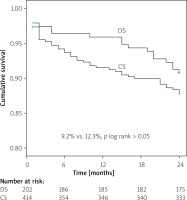
Figure 2
Kaplan-Meier curve estimate of freedom from stent thrombosis (ST) at 24 month follow-up
DS – direct stenting, CS – conventional stenting.
DS was found to be a predictor of ST at 2 years (hazard ratio (HR) = 0.07, 95% confidence interval (CI): 0.01–0.66, p = 0.021) in univariate Cox analysis. However, when adjusted for confounding factors, DS was not an independent predictor of ST at 2 years in multivariate Cox analysis (HR = 0.24; 95% CI: 0.05–1.04; p = 0.058).
Discussion
This is the first study to include STEMI patients with small vessel coronary artery disease who were treated with DS or CS and report their long-term outcomes. The main findings of the current study are as follows: First, the rate of MACEs, the primary end-point, was similar in the direct stenting group as compared with that in the patients treated with conventional stenting. Second, there were no statistically significant difference in TLR, TVR, MI, in-hospital death and in-hospital TLR. However, conventional stenting was associated with a significantly higher stent thrombosis rate at 2-year follow-up and also higher procedural complication. DS was not found to be an independent predictor of ST at 2 years in multivariate analysis. Moreover, DS was associated with a shorter procedure and fluoroscopy time, as well as lower contrast volume and postprocedural TIMI III flow.
DS is defined as the technique of coronary stent implantation without predilatation by balloon. To perform direct stenting, it is mandatory to visualize the length of the culprit lesion and the diameter of the vessel. However, in a STEMI patient, TIMI flow is most often ≤ 1 before wire placement [14]. During primary PCI, patients who had TIMI flow of ≥ 2 at initial injection or after wire placement are suitable for direct stenting. The direct stenting technique has a number of theoretical advantages. Prospective studies and meta-analyses have consistently demonstrated significant benefit of DS in terms of safety, procedural outcomes, MACE rate and mortality [15]. Superior clinical outcomes associated with DS may be driven by reduced wall damage and inflammatory response from balloon predilation [16], and fewer intimal dissections [17]. Balloon predilation before stenting is associated with higher risk of distal embolization and microvascular occlusion, which is associated with more reperfusion failure and lower probability of final TIMI 3 flow [3, 18]. In our study, a relatively high incidence of procedural complications and lower TIMI III flow rate were observed in the conventional stent group versus the direct stent group, compatible with previous results. However, underestimation of true vessel size, failure to cross the lesion, non-dilatable lesions, inadequate stent expansion and late stent malapposition were possible limitations of DS [19].
Routine use of manual thrombus aspiration in primary PCI resulted in improved myocardial reperfusion and reduced 1-year cardiac mortality in the TAPAS study [20]. However, the efficacy of routine thrombus aspiration could not be confirmed in either the TASTE [21] or the TOTAL [22] trial, prompting international guidelines to advise its use in selected patients only [23]. In a recent study, Mahmoud et al. reported that clinical outcomes and myocardial reperfusion measures did not differ significantly between DS and CS and there was no interaction with thrombus aspiration [24]. In this study, we used thrombus aspiration only in selected patients, and thrombus aspiration was used at a similar rate in both groups.
The results of studies investigating the usefulness of the DS strategy in terms of clinical outcomes are contradictory. In recent years, several meta-analyses comparing DS and CS have been published. However, these studies have not been performed in STEMI patients or in small vessel coronary artery disease. Therefore, the outcome rates in our study are important in terms of being the first data in this patient group. Magalhaes et al. reported significantly lower MACE and TLR rates following DS as compared with CS in a meta-analysis of elective procedures [15]. In another meta-analysis, myocardial infarction was significantly reduced with direct stenting compared with conventional stenting, but the target-vessel revascularization rate was similar between groups [6]. Moreover, Alak et al. found a significant reduction in the risk of in-hospital cardiovascular death, but no significant differences were observed in myocardial infarction or target lesion revascularization [25]. Different results were found in each of these three metaanalyses, which might have resulted from studies with different methodologies in different patient groups. They included many old treatment elements, including antiplatelet therapy with ticlopidine and implantation of mostly bare metal stents.
In the literature, there are few studies comparing DS and CS in terms of clinical outcomes in STEMI patients. Kalayci et al. found that DS in primary PCI was associated with better postprocedural angiographic results and long-term survival; however, the DS group had similar in-hospital and long-term mortality to matched patients in the CS group [26]. In another study, direct stenting compared to conventional stenting was associated with a significantly lower rate of all-cause death and stroke, but there were non-significant differences in target lesion revascularization, myocardial infarction, stent thrombosis and major bleeding at 1-year follow-up [27]. McCormick et al. reported that patients receiving DS had reduced mortality at 30-day and 1-year follow-up [28]. In the EUROTRANSFER registry, direct compared with conventional stenting resulted in significantly higher rates of postprocedural TIMI grade 3 flow, lower risk of no-reflow and significant reduction in 1-year mortality [29]. In the present study, the rate of edge dissection was lower and the rate of TIMI III flow was higher in the DS group. However, there was no difference in terms of no-reflow between groups. In-hospital death and TLR at 2 years were also lower in the DS group, but it did not reach significance. The rate of stent thrombosis is higher in patients with small vessel CAD as compared with those with non-small vessel CAD in the literature [30]. In the previous studies ST rates were found to be similar in DS and CS groups in non-small vessel CAD. In the current study, although the stent thrombosis rate was higher in the CS group in STEMI patients with small-vessel CAD, DS was not found to be an independent predictor of ST at 2 years in multivariate analysis. Increased stent thrombosis in the CS group may be associated with the use of longer and smaller diameter stents in this group. In addition, the suboptimal procedural result (TIMI flow grade < 3) is a predictor of ST, and in our study the TIMI III flow rate was lower in the CS group.
Limitations
Several limitations of the present study should be acknowledged. First, the present trial was a single centre pilot study representing the experience of only one hospital and the results can only be hypothesis-generating. Second, only about 25% of our patients received follow-up coronary angiography according to clinical indications, and the potential bias related to the incomplete angiographic follow-up might have had a substantial impact on the analytic results. Third, a bias cannot be excluded as to which patients seemed suitable for direct stent implantation.
Conclusions
As in our study, there is no other study comparing DS and CS in terms of clinical outcomes in small-vessel CAD patients with STEMI. Direct stenting without predilation in selected lesions seems to be a safe and successful procedure that provides a lower stent thrombosis and procedural complication rate and potential advantages as savings in procedural time, contrast load, and shortened radiation exposure time. However, larger and randomized studies with longer follow-up are mandatory before routine clinical use is recommended.








