Introduction
Primary sclerosing cholangitis (PSC) is an uncommon chronic liver disease characterised by inflammation of unknown origin that induces fibrosis and strictures of the bile ducts, causing cholestasis and, in the long term, complete stenosis leading to liver cirrhosis [1, 2]. For patients with severe form of the disease, the recommended treatment is liver transplantation (OLTx) [3]. Following liver transplantation, only about 1/5 of patients will experience PSC recurrence in the transplanted liver, indicating that transplantation alters the natural history of PSC [4]. In patients with PSC co-occurring with ulcerative colitis (UC), there is a significantly more extensive involvement of the large intestine. Additionally, the terminal sections of the small intestine are more frequently affected, resulting in what is known as backwash ileitis. This occurs due to the retrograde flow of inflammatory mediators through the inefficient ileocecal valve. Moreover, there is lower inflammatory activity observed in the rectal mucosa, leading to a more frequent occurrence of the right-sided UC phenotype [3, 5]. Recent studies have demonstrated that patients requiring liver transplantation due to PSC with concomitant UC more commonly report an oligosymptomatic course of UC, with fewer exacerbations and a reduced need for immunosuppressive treatment. However, these patients who undergo liver transplantation due to PSC are significantly more likely to require abdominal surgical interventions, occurring even 3 times more frequently. Furthermore, they are at an increased risk of colorectal cancer. This may suggest that a more severe course of PSC may somehow have a protective effect on UC, which ends after liver transplantation (OLTx) [6]. Moreover, the presence of UC is associated with an increased risk of faster progression of PSC [7]. This may be due to several reasons – it was found that in the blood of patients with UC coexisting with PSC, a higher ratio of CD4 to CD8 lymphocytes was present than in the blood of patients with UC without PSC. Another possibility is the postulated parenteral expression of adhesion proteins and chemokine receptors in the liver, which may result in the recruitment of lymphocytes from the intestine via portal circulation, which will decrease their concentration in the intestine wall, thereby reducing colonic inflammation and worsening the course of PSC [8, 9]. There is also evidence that the presence of an intact intestine at the time of liver transplantation increases the risk of PSC recurrence in the transplanted liver, which may confirm the relationship between the course of PSC and UC activity [10]. However, data on the course of UC after liver transplantation for PSC are unclear: in some studies, no or little protective effect of transplantation was observed, although some authors suggest that it may be a matter of the selection of immunosuppressive treatment [11–13]. On the other hand, other studies have demonstrated worsening of UC after liver transplantation due to PSC [14, 15]. In the research of Jorgensen et al. it was also shown that liver transplantation due to PSC may have a positive effect on the course of inflammatory bowel diseases, without specifying UC or Crohn’s disease; however, 86% of patients included in the study suffered from UC [16]. In the studies by Villamil et al. a silent course of UC was found in the majority of patients after liver transplantation due to PSC, although there was a group in which UC symptoms worsened and a high probability of dysplasia or colorectal cancer was observed [17]. Currently, there seems to be no clarity about the course of UC in patients, who underwent liver transplantation due to PSC.
Aim
Here we present a cross-sectional study designed to evaluate colonic inflammation in patients with UC concomitant to PSC (PSC-UC) before and after transplantation.
Material and methods
Patients
The study was approved by the Bioethics Committee of the Pomeranian Medical University in Szczecin, Poland. Databases of all patients who underwent liver transplantation and all patients attending the Hepatological Outpatient Clinic were reviewed. Patients older than 18 years, with PSC concomitant with UC treated in the Hepatological Outpatient Clinic, Hepatology Ward, or Gastroenterology and Internal Diseases Ward of Arkońska Hospital were included in the study. Patients were divided into 2 cohorts: those who underwent liver transplantation due to PSC, and patients without liver transplantation. Cases with PSC alone, unclear colitis, transplantation due to reasons other than PSC, and patients with other active inflammatory conditions were excluded from the study. Patients currently undergoing flare of UC were either excluded from the study or enrolled on the next visit. Finally, 25 patients who underwent liver transplantation due to PSC, with concomitant UC, and 38 patients with PSC concomitant to UC, who did not undergo liver transplantation, were enrolled to the study. All study participants had routine PSC and UC treatment provided in accordance with the standard of care.
Data collection
All included patients were approached during standard follow-up visits in the Hepatological Outpatient Clinic; patients had the study design and the aim explained, and gave their written consent. Subsequently, the UC severity was established using the Clinical Activity Index (CAI), as proposed by Rachmilewitz [18]: number of stools per week, blood in the stool, the general condition of the patient, abdominal pain, presence of the blood in the stool, body temperature, extracolonic symptoms, erythrocyte sedimentation rate, and haemoglobin concentration levels were recorded in a questionnaire (Table I).
Table I
Clinical Activity Index (CAI) according to Rachmilewitz
Stool samples were collected, labelled, and stored at –30°C. Calprotectin levels were measured using the ELISA EUROIMMUN AG Calprotectin test. White blood cell count (WBC), C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) were tested in blood samples drawn from the patients, using routine laboratory analysis.
Statistical analysis
Statistical analysis was conducted using IBM SPSS Statistics, version 27.0 (IBM, Sheffield, UK) and Microsoft Excel. Categorical variables were presented as numbers and percentages. Continuous variables were presented as mean and 95% confidence intervals and standard deviation or median with interquartile range (IQR). The Shapiro-Wilk test was used for checking variable normality. Sample mean values before and after OLTx were compared using one-way ANOVA. The odds ratio was calculated according to Altman. P-values of 0.05 or less were considered statistically significant.
Results
The study group consisted of 63 patients: 25 men and 38 women, with a mean age of 44 years (IQR: 31.75–57.25). Twenty-five (40%) of the patients underwent liver transplantation due to PSC. In the group after OLTx the median time from transplantation was 62 months (IQR = 87). Further study group characteristics are summarised in Table II. In both groups no significant difference between CAI levels were shown – mean was 1.06 vs. 0.92 after and without OLTx, respectively (p = 0.33). The study showed a significant rise in intestinal inflammation: calprotectin levels were significantly higher in the group after OLTx than in the group without OLTx – mean levels were 474.07 ng/ml (95% CI: 235.42–712.72) and 180.45 ng/ml (95% CI: 18.11–342.79), respectively (p = 0.024), which translates to a 163% rise in mean levels (Figure 1). Calprotectin levels exceeded the upper limit of normal (defined as 200 ng/l) in 66% of liver recipients and in 18% of patients in the group that did not undergo transplantation (OR = 9.33, p = 0.011). In systemic inflammation markers the mean CRP level was shown to be significantly higher in the group after liver transplantation: 11.01 mg/l (95% CI: 6.78–15.23) vs. 6.54 mg/l (95% CI: 3.98–9.09) in the group without OLTx (p = 0.030) (Figure 2). Also, WBC levels were significantly higher in the transplantation group than in non-transplanted patients – 7.58 K/ml (95% CI: 5.15–9.12) vs. 5.72 K/ml (95% CI: 5.15–6.28) (p = 0.006) (Figure 3). In the group of patients after OLTx, ESR was also higher than in the group that did not receive an organ transplant: 21.13 mm/h (95% CI: 12.65–29.60) vs. 14.87 mm/h (95% CI: 8.68–21.06), although the statistical significance threshold was not reached: p = 0.12 (Figure 4). All laboratory findings are summed up in Table III. OLTx had a greater, and statistically significant, impact on intestinal rather than systemic inflammation parameters – mean faecal calprotectin values were higher by 163% in the post-OLTx group than in the group without transplantation, whereas the mean CRP level increased by 68%, and WBC by 32%. Also, ESR showed a similar increase in the post-OLTx group (41%), but the effect was not statistically significant.
Table II
Clinical characteristics of patients with primary sclerosing cholangitis (PSC) concomitant with ulcerative colitis (UC) and patients with PSC concomitant with UC who underwent liver transplantation due to PSC
Figure 1
Comparison of faecal calprotectin levels in patients with primary sclerosing cholangitis (PSC) concomitant with ulcerative colitis (UC) and patients with PSC concomitant with UC who underwent liver transplantation due to PSC
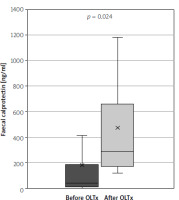
Figure 2
Comparison of C-reactive protein levels in patients with primary sclerosing cholangitis (PSC) concomitant with ulcerative colitis (UC) and patients with PSC concomitant with UC who underwent liver transplantation due to PSC
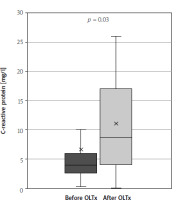
Figure 3
Comparison of white blood cell count in patients with primary sclerosing cholangitis (PSC) concomitant with ulcerative colitis (UC) and patients with PSC concomitant with UC who underwent liver transplantation due to PSC
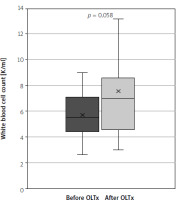
Figure 4
Comparison of erythrocyte sedimentation rate in patients with primary sclerosing cholangitis (PSC) concomitant with ulcerative colitis (UC) and patients with PSC concomitant with UC who underwent liver transplantation due to PSC
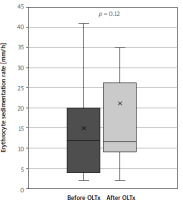
Table III
Laboratory findings of patients with primary sclerosing cholangitis (PSC) concomitant with ulcerative colitis (UC) and patients with PSC concomitant with UC who underwent liver transplantation due to PSC
Discussion
The course of UC after liver transplantation due to PSC remains unaccountable. In this study we demonstrated that liver transplantation due to PSC greatly increases both systemic and intestinal inflammation. We found that faecal calprotectin was significantly higher in the post-OLTx group vs. the non-transplant group, i.e. by 163% – mean values were 474 ng/ml and 180.45 ng/ml, respectively (p = 0.02). The post-OLTx group also had higher CRP concentration (11.01 mg/l vs. 6.54 mg/l, p = 0.03) and higher WBC (7.58 K/ml vs. 5.72 K/ml, p = 0.006). ESR was also higher in the post-OLTx group (21.13 mm/h vs. 14.87 mm/h, p = 0.122). Despite the result not being statistically significant, it is consistent with other findings presented in this study. The impact of OLTx seems to be greater on intestinal than on systemic inflammation markers. This may be explained by the presence of active PSC in the group of patients who did not receive OLTx. UC seemed to remain quiescent in the presence of active PSC – in only 18% of non-transplant patients we found calprotectin levels that exceeded the upper limit of normal (defined as 200 ng/l), whereas in the post-OLTx group calprotectin levels exceeded the upper limit of normal in 66% of patients (OR = 9.33, p = 0.011). The effect may be greater because in some studies it was shown that faecal calprotectin may be elevated in the sole presence of PSC, and it has also been tested as a marker of biliary inflammation [19, 20]. Such an effect is not certain, though; in some cohorts the presence of PSC has not altered calprotectin levels in UC patients [21]. As calprotectin is highly sensitive to intestinal inflammation, the predominance of high faecal calprotectin over systemic inflammation marker increases the likelihood of the results being UC related [22]. One of the hypotheses of PSC pathogenesis suggests that lymphocytes activated in the bowel of inflammatory bowel disease patients translocate to the liver and cause disease progression [9]. This may concur with our study findings – the presence of active PSC causes a recruitment of intestinal activated lymphocytes, mitigating the course of UC; on the other hand, after transplantation, when no chemoattractants are present in the transplanted liver, activated lymphocytes remain in the colonic wall, causing further damage. We did not see any influence of OLTx on clinical symptoms of UC, as defined by CAI – the mean was 1.06 vs. 0.92 after and before OLTX, respectively (p = 0.33), but patients undergoing active flares were excluded from the study. Calprotectin is highly specific and superior to the laboratory findings, when assessing intestinal inflammation [23], so according to our study one can state that OLTx due to PSC may cause a significant and capital impact on intestinal inflammation due to UC.
This study has several limitations: the study group was not large enough to determine statistical significance of all tested parameters. It must be stated that assembling a group of patients with PSC-UC after liver transplantation due to PSC may be a challenge, and multicentre studies may be necessary. It must also be stated that in the study additional inflammation markers could be used, which would have provided additional information: for example, the peripheral blood neutrophil-to-lymphocyte ratio has proven to be a valuable biomarker for predicting disease severity in IBD patients [24]. Another novel inflammation marker that has shown usefulness in monitoring patients after colorectal surgery, which may also provide more information, is butyrylcholinesterase [25–27]. A further limitation is the fact that the study was cross-sectional in nature; therefore, we only can state that the parameters are higher in the group post-OLTx. The significance of this effect may be crucial to the patients’ treatment, so appropriate prospective studies should be done. This study seems to confirm a great amount of previously conducted research. Dvorchik et al. described worsening UC symptoms after liver transplantation due to PSC and a 3.1-fold increase in the risk of needing a proctocolectomy [28], which is consistent with our findings of greatly increased intestinal inflammation. Worsening of UC symptoms and increased risk of surgery were confirmed in the other research by Papatheodoridis et al. [15]. Also, the disease activity described by Mayo score was shown to be greater after liver transplantation in the Hungarian population [14].
Our study is in contrary to many previously published papers – Jorgensen et al. in a cross-sectional study showed no impact of liver transplantation on faecal calprotectin levels, but the results were not statistically significant [16]. Also, endoscopically obtained data from a large cohort study confirmed the worsening of UC after PSC-related OLTx [29]. Some studies provide conflicting results – Osiecki et al. saw no effect on UC course after OLTx due to PSC, but the research was conducted on a group of 17 patients; the authors stated that the course of UC was not aggravated by OLTx for PSC, but no statistical significance was obtained [30]. Even larger studies have described little to no worsening of UC symptoms after PSC-related OLTx, but no laboratory differences were tested [12]. Similarly, there is evidence that the number of flares did not increase [11]. Because it is the only study to date providing significant results on the intestinal markers of UC activity in the group of patients with UC after liver transplantation due to PSC, one can assume that the intestinal disease activity is intensified, which may translate to a need for higher doses and longer treatment when a UC flare is present, as well as increased doses when the disease remains quiescent. It has also been found that in patients with elevated calprotectin levels, colonic dysplasia and colorectal cancer may be more frequent [31, 32]. This may implicate the need for more frequent colonoscopies in this group of patients.
Conclusions
Our cross-sectional study of the impact of liver transplantation on intestinal and systemic inflammation markers in patients with colitis ulcerosa concomitant with primary sclerosing cholangitis has shown that despite no differences in clinical symptoms, after liver transplantation due to PSC there is a significant rise in both systemic and intestinal inflammation markers. Calprotectin levels showed to be impacted the most by liver transplantation amongst tested parameters, which confirms the intensification of intestinal inflammation in UC patients who underwent liver transplantation due to PSC. This implies that intensification of UC treatment may be needed, despite the lack of worsening of clinical symptoms.










