Introduction
Periampullary diverticulum (PAD) refers to an outpouching of duodenal mucosa or submucosa, typically caused by a defect in the muscular layer, and it occurs within 2–3 cm of the major papilla [1, 2]. It is usually discovered incidentally during endoscopic retrograde cholangiopancreatography (ERCP) in 3–32% of patients [3–5]. Studies have shown that PAD is less prevalent in patients under 40 years of age, with its incidence increasing with age [4, 6, 7].
The formation of PAD is associated with the progression of duodenal dysmotility, which is caused by high intraduodenal pressure and the progressive weakening of the intestinal smooth muscle [8]. Although PAD is often asymptomatic [4, 9], it can lead to sphincter of Oddi dysfunction (SOD), mechanical compression of the common bile duct (CBD), impaired biliary drainage, and the development of CBD stones, gallstones, pancreatitis, and acute cholangitis [2, 4, 10]. In rare cases, it may also lead to bleeding, perforation, and even cancer [11].
Periampullary diverticulum can be subclassified based on the location of the papilla. Boix et al. classified it into three groups: type I when the papilla is inside the diverticulum, type II when it is at the edge of the diverticulum, and type III when it is outside the diverticulum [12]. Panteris et al. simplified the classification into two groups: type A when the papilla is on the edge or within 2 cm of the edge of the diverticulum (types II and III), and type B when it is inside the diverticulum or between two adjacent diverticula (type I) [13].
The impact of PAD on ERCP remains controversial, with some studies indicating no effect on success rates [6, 14], while others suggest that it may increase the difficulty of ERCP and the risk of complications [5, 9]. Further studies are needed to determine the full extent of PAD’S influence on ERCP.
Material and methods
Patients and study design
This study enrolled 400 male and female patients aged 18 years and above who underwent therapeutic ERCP for CBD stones at the National Liver Institute (NLI), Menoufia University, between October 2020 and September 2022. The presence of CBD stones was confirmed through abdominal computed tomography (CT) or magnetic resonance cholangiopancreatography (MRCP). Exclusion criteria included patients under 18 years of age, a history of previous endoscopic sphincterotomy (EST), prior gastrointestinal tract (GIT) surgery, severe bleeding disorders, or concomitant pancreatic or biliary malignancies.
At the outset of the study, patient demographics and characteristics were recorded. All patients underwent comprehensive physical examinations, vital signs assessments, and a series of tests including complete blood count, liver and kidney function tests, amylase, lipase, electrocardiogram, abdominal ultrasound, and abdominal CT or MRCP.
Patients were categorized into two groups: the PAD group (comprising 200 patients with PAD detected during ERCP) and the non-PAD group (consisting of 200 patients without PAD detected during ERCP). The PAD group was further divided into three types based on the location of the major papilla: type 1 (major papilla inside PAD – 81 patients), type 2 (major papilla at the edge of PAD – 66 patients), and type 3 (major papilla outside of PAD – 53 patients).
Methods and procedures
All ERCP procedures were performed by highly experienced endoscopists using standard side-viewing duodenoscopes (Pentax EPK-i5000). In our center we rely on good hydration rather than anti-inflammatory suppositories as a preventive measure for pancreatitis. Conventional selective cannulation was attempted using a cutting guide wire. In cases where selective cannulation failed, procedures such as precut or trans-pancreatic sphincterotomy with coagulation were performed. After cannulation of the CBD and cholangiography, EST alone or small EST combined with endoscopic papillary balloon dilation (EPBD) was carried out based on the number and diameter of stones, the position of the papilla, and the length of the side. Following stone removal, balloon cholangiography was performed to confirm the complete removal of stones.
Data recorded included the presence of PAD, the location of the papilla relative to PAD, CBD diameter, number and diameter of CBD stones, method of cannulation, procedure time, and the success rate of CBD clearance.
After the procedures, all patients were monitored for post-ERCP complications, such as bleeding, pancreatitis, cholangitis, or perforation.
Study outcomes
The primary endpoint of this study was to investigate the impact of PAD on technical success, the rate of complications, and procedure time in therapeutic ERCP. The secondary endpoint was to assess the influence of the type of PAD on these parameters.
All cases with unsuccessful cannulation by ERCP were done by an endoscopic ultrasound (EUS) guided transmural biliary approach and successfully managed.
Statistical analysis
Data were entered into a computer and analyzed using IBM SPSS software package version 20.0 (Armonk, NY: IBM Corp). Categorical data were presented as numbers and percentages. The χ2 test was used to investigate associations between categorical variables, with Monte Carlo correction applied when expected cell counts were less than 5. For continuous data, normality was assessed using the Kolmogorov-Smirnov test. Normally distributed quantitative variables were expressed as a range (minimum and maximum), mean, standard deviation, and median. Student’s t-test was employed to compare two groups, and the one-way ANOVA test was used for comparing the three studied groups. For non-normally distributed quantitative variables, the Mann-Whitney test and Kruskal-Wallis test were used to compare two groups and different groups, respectively. The significance level was set at 5%.
Results
Baseline characteristics of patients
The study included 400 patients, with 200 in the PAD group (group 1) and 200 in the non-PAD group (group 2). In the PAD group, there were 127 (63.5%) males and 73 (36.5%) females, with a mean age of 59.2 ±6.6 years. In the non-PAD group, there were 124 (62%) males and 76 (38%) females, with a mean age of 58.3 ±6.9 years. The study found no significant differences between the PAD and non-PAD groups regarding age (p = 0.185) or sex (p = 0.756) (Table I).
Table I
Comparison between the two studied groups based on demographics, therapeutic outcomes, and complications
When comparing the three types of PAD, there were 51 (63%) males and 30 (37%) females with type 1, with a mean age of 58 ±7 years. There were 41 (62.1%) males and 25 (37.9%) females with type 2, with a mean age of 59.7 ±5.9 years. There were 35 (66%) males and 18 (34%) females with type 3, with a mean age of 60.3 ±6.8 years. The study found no significant differences between the types of PAD regarding age (p = 0.113) or sex (p = 0.900) (Table I).
Effect of PAD on size and number of stones and CBD diameter
In the PAD group, there were 1–5 stones with a mean of 1.5 ±0.8 stones, a mean largest stone size of 12.4 ±3.5 mm, and a mean CBD diameter of 15.1 ±3.6 mm. In the non-PAD group, there were 1–4 stones with a mean of 1.5 ±0.8 stones, a mean largest stone size of 12.6 ±3.2 mm, and a mean CBD diameter of 15.1 ±3.4 mm. The study found no significant differences between the PAD and non-PAD groups regarding the number of stones (p = 0.536), stone size (p = 0.593), or CBD diameter (p = 0.998) (Table II).
Table II
Relationship between papillary location and demographic data, therapeutic outcomes, and complications in group 1 (n = 200)
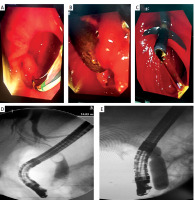
When comparing the three types of PAD, there were 1–4 stones with a mean of 1.56 ±0.8 stones, a mean largest stone size of 12 ±3.2 mm, and a mean CBD diameter of 14.7 ±3.3 mm in type 1 patients. In type 2 patients, there were 1–4 stones with a mean of 1.41 ±0.7 stones, a mean largest stone size of 12.9 ±3.5 mm, and a mean CBD diameter of 15.2 ±3.3 mm. In type 3 patients, there were 1–5 stones with a mean of 1.43 ±0.9 stones, a mean largest stone size of 12.5 ±4 mm, and a mean CBD diameter of 15.4 ±4.2 mm. The study found no significant differences between the types of PAD regarding the number of stones (p = 0.288), stone size (p = 0.266), or CBD diameter (p = 0.620) (Table II, Figure 1).
Discussion
In our study, we aimed to evaluate the success and safety of ERCP procedures in patients with PAD. While prior research has produced differing results on this topic, our study provides valuable insights.
Contrary to some previous studies [6, 15], our research found no statistically significant difference in age between patients with PAD and those without (p = 0.185). It is worth noting that PAD is infrequently observed in patients under 40 years old. Zheng et al. reported a higher prevalence of PAD in individuals over 60, suggesting a significant correlation between PAD and age [16]. The higher incidence of PAD in older age may be attributed to age-related factors such as muscular and intestinal wall deterioration, weakened intestinal contractile tone, slow peristalsis, and abnormal retention of intestinal contents [17].
Gender-based analysis revealed conflicting findings in past research [13, 17, 18]. However, our study found no significant difference between the presence of PAD and gender (p = 0.756). Karaahmet et al. found a higher prevalence of PAD in females [5], and Sfarti et al. noted a weak association with female gender [19]. In contrast, studies by Mohammad et al. [3] and Örmeci et al. [7] indicated that the prevalence of PAD was not related to gender, while Zheng et al. reported a higher prevalence of PAD in males [16].
When analyzing the types of PAD, our study found no significant differences in age (p = 0.113) or gender (p = 0.900) among different types. This aligns with Hu et al.’s findings [20] but contrasts with studies by Kim et al. [21] and Chen et al. [4], which found that PAD type 1 was significantly more common in older patients.
Concerning the association between PAD and stone size and CBD diameter, previous studies have reported mixed results. Some indicated significant associations between the presence of PAD and larger stones or larger CBD diameters [22–24], while others, such as Panteris et al., found no such associations [13]. In our study, no significant associations were observed between PAD and stone size (p = 0.593), the number of stones (p = 0.536), or CBD diameter (p = 0.998), and these factors were not significantly affected by different types of PAD.
Historically, PAD was considered a primary factor contributing to ERCP failure, with associations with higher cannulation failure, lower CBD clearance rates, and longer procedure times [2, 6, 11, 25]. However, more recent research has challenged this notion, with findings indicating that the presence of PAD does not significantly affect ERCP success rates [1, 5, 15]. This inconsistency may be attributed to variations in cannulation techniques, endoscopist experience, patient characteristics, and a lack of adjustment for these variables in patient and control groups [13].
Our study confirmed that the presence of PAD had no significant effects on the difficulty of cannulation, or the methods used (p = 0.099), procedure times (p = 0.705), or the rate of CBD clearance (p = 0.782). Notably, Chen et al. reported no significant differences in the success rate of selective cannulation between patients with and without PAD but did find a significantly lower CBD clearance rate in patients with PAD [4]. Corral et al. also found that the success rate of ERCP was similar for patients with or without PAD, with shorter procedure times in patients with PAD but similar fluoroscopy times for PAD and non-PAD groups [14].
Classifying PAD into three types based on the location of the papilla, our study revealed that PAD type 1, where the papilla is inside the diverticulum, was associated with significantly more difficult cannulation and a significant failure of the selective cannulation technique (p < 0.001). It was also linked to a significantly lower rate of CBD clearance (p < 0.001) compared to other types. This aligns with the findings of Lobo et al. and Panteris et al. [8, 13] and Chen et al. [4], who reported that cannulation was more challenging in type 1 PAD.
It is important to note that Mu et al., in a meta-analysis, found that an interdiverticular papilla (IDP) was associated with a higher rate of cannulation failure [26], and Tabak et al. reported that cannulation in type 1 PAD was more challenging and associated with longer cannulation times compared to other types [27]. This may be attributed to the difficulty in locating the papillary orifice and predicting the bile duct direction in this type of PAD [28].
In contrast to our study, Tyagi et al. [17] and Katsinelos et al. [24] reported that the location of the papilla had no effect on cannulation success and no differences in final cannulation rates or methods between different types of PAD.
Endoscopic retrograde cholangiopancreatography is an invasive procedure, and complications are a possibility. Our study documented only post-ERCP pancreatitis (PEP) and minor bleeding, with no instances of perforation, cholangitis, or major bleeding. The incidence of PEP following endoscopic papillary balloon dilation (EPBD) ranges from 5% to 19.8% [29]. Balloon dilatation can cause spasm and compression of the distal pancreatic duct, restricting the flow of pancreatic juice and leading to pancreatitis [30]. Combining small endoscopic sphincterotomy (EST) with EPBD can reduce the risk of PEP [31].
PAD can increase the risk of bleeding due to repeated cannulation attempts, the need for additional EPBD or EST, and deformation of adjacent blood vessels [32]. While there have been conflicting reports on post-ERCP complications in patients with PAD, our study found no significant difference between patients with PAD and those without regarding post-ERCP complications (p = 0.371).
Some studies have reported higher rates of PEP in patients with PAD [33], while others have shown similar rates of PEP in patients with and without PAD [3, 7, 19]. Some studies have even suggested that PAD might have a protective effect against PEP [32, 34]. In contrast to our findings, Parlak et al. and Chen et al. reported significantly higher rates of PEP in patients with PAD [15, 35]. However, Hu et al. found that the rate of PEP did not increase in patients with PAD, with all cases of pancreatitis being mild and recovering with conservative treatment within 3 days [20]. A meta-analysis by Jayaraj in 2018 indicated that the presence of PAD was not a risk factor for PEP [36].
Consistent with our results, Hu et al. [20], Park et al. [28], and Jayaraj et al. [36] reported that the rate of ERCP-related bleeding was similar in patients with and without PAD. However, in contrast to our findings, Chen et al. [15] and Karaahmet et al. [5] reported a higher incidence of bleeding in patients with PAD. Conversely, our study investigated the impact of various types of PAD and identified that PAD type 1 was significantly associated with an increased rate of post-ERCP pancreatitis (PEP) (p = 0.002). However, when considering bleeding, no significant differences were observed among different types of PAD (p = 0.286). Kim et al. [37] and Tabak et al. [27] concurred by reporting a higher rate of PEP in patients with PAD type 1 than in those with other types. Tabak et al. [27] additionally noted a higher rate of bleeding in type 3.
The elevated incidence of PEP in type 1 PAD can be explained by the specific location of the papilla within the diverticulum, making cannulation and predicting the bile duct direction more challenging. This increased complexity may lead to pancreatitis due to unnecessary contrast medium injection or manipulation of the pancreatic duct [37]. In contrast to our findings, Chen et al. [15] and Mu et al. [26] reported no significant association between the type of PAD and the rate of PEP.
Our study had several advantages. Firstly, all patients were more homogeneous as they had the same indication for ERCP. Secondly, it was a prospective study, allowing for precise recording of procedure details, fluoroscopy times, and post-ERCP complications.
However, our study had certain limitations. Firstly, it was conducted at a single center, potentially limiting the generalizability of the results. Secondly, all procedures were performed by highly experienced endoscopists, and as such, these findings may not be directly applicable to less experienced practitioners.