Introduction
Rectal cancer treatment has advanced over the past few decades, and as a result, the oncological prognosis has improved in terms of the reduced local recurrence (LR) rate and increased survival [1–3]. It has been anticipated that a potential mechanism of local recurrence in colorectal anastomosis involves the implantation of exfoliated malignant cells [4, 5]. Experimental research has demonstrated that colorectal cancer cells shed viable cells into the gut lumen that are potential implantable clones [6, 7]. Consequently, it has been suggested that rectal washout be done prior to cross-stapling during anterior resection [8].
Many colorectal surgeons use intraoperative rectal washing, which was developed in 1951 by Goligher et al. to limit the amount of free cancer cells [9]. The American Society of Colon and Rectal Surgeons’ 2005 practice guidelines for the therapy of rectal cancer, however, came to the conclusion that there were insufficient data to support the recommendation of intraoperative rectal washout to prevent local recurrence [10]. The results of previous meta-analyses, which analyzed research but did not assess it carefully and included too-old studies, are completely different from those of the present [11–13].
Over the past few decades, studies comparing the effectiveness and safety of rectal washout versus no rectal washout have been done, with usually inconsistent results. The main objective of this systematic review is to investigate research studies that assessed rectal washout versus no washout in rectal cancer for the prevention of local recurrence.
Material and methods
Data collection and methodology
We thoroughly searched PubMed, Cochrane Library, and SCOPUS for articles published between 2005 and July 2023. We carried out this research in compliance with PRISMA recommendations. In this study, local rectal cancer recurrence was compared between rectal washout and no rectal washout. Animal experimentation, case studies, reviews, and letters were not however included. The MINORS for nonrandomized controlled trials was used to assess the quality of the studies that were included [14].
PICO
Population: SCOPUS, Cochrane Library, and PubMed databases for articles available from January 2005 to July 2023.
Intervention: Rectal washout providing significant results with no local recurrence.
Comparison: We explored studies comparing rectal washout versus no washout for rectal cancer and focused on the probability of local recurrence.
Outcome: It helps to enhance and improve quality of life in some way to have significant results in rectal washout with a 5-year follow-up compared to not having rectal washout.
Inclusion and exclusion criteria
The terms “rectal cancer”, “washout” and “local recurrence” were used in our research. Humans and English-language content only were included in our search. Studies that did not compare rectal washout versus no washout and those carried out in languages other than English were also excluded. Those that did not meet the inclusion criteria for rectal cancer, local recurrence or distant metastasis were also excluded.
Statistical analysis
The statistical meta-analysis was conducted using RevMan 5.4. The confidence interval (Cl) was set at 95% for the summary statistics and they are ordered in accordance with the descriptive analysis. Results are shown for dichotomous data using odd ratios (OR) and 95%Cl according to the Mantel-Haenszel method. We employed Q statistics to evaluate the treatment effects of heterogeneities, I2 was evaluated for total variation studies, and statistical significance was defined at p < 0.05. The heterogeneity (I2 > 75% indicates substantial heterogeneity, p < 0.1 indicates significant heterogeneity) was assessed using the Q test and if there was no heterogeneity or it was minimal, the fixed effect model was employed; otherwise, the random effect model was applied [15].
Results
Patient characteristics
With the search terms “rectal cancer”, “washout”, and “local recurrence” a total of 324 articles from PubMed, Scopus, Medline, and Cochrane Library were identified. Following the screening of titles and abstracts, 109 articles were eliminated due to duplication. A total of 8 full-text articles were then retrieved, of which 69 were unrelated and 31 were omitted because they contain “no comparison between rectal washout and no rectal washout”, “case reports”, “conference study”, “literature”, and “editorial” (Figure 1).
We identified this in our research, which included 19,855 participants from 8 studies. The articles contained randomized control trials (RCT) and non-randomized control trials (NRCT), with 15127 patients undergoing rectal washout (RW) and 4728 receiving non-rectal washout (NRW). As shown in Table I [16–23], an average score of 22 (within a range of 19–23) was chosen to illustrate the significance of involving research that rigorously satisfies MINORS quality criteria.
Table I
Characteristics of the studies included in the meta-analysis (RW/NRW)
| Study ID | Study type | Washout solution | Total | Rectal washout (RW) | No rectal washout (NRW) | LRR WO% | LRR NWO% | Age (n) (WO/NWO) | Follow-up | MINOR |
|---|---|---|---|---|---|---|---|---|---|---|
| Terzi 2006 [16] | NRCT | Povidone-iodine 5% | 96 | 38 | 58 | 7.8 | 3.4 | 59.6/61.8 | 36 | 19 |
| Kodeda 2010 [17] | RCT | NR | 4600 | 3749 | 851 | 6 | 9.9 | 69/70 | 60 | 22 |
| Xingmao 2013 [18] | NRCT | NaCl 0.9% | 144 | 65 | 79 | 4.3 | 6.6 | 56.3/59 | 60 | 20 |
| Jörgren 2017 [19] | RCT | NR | 1188 | 686 | 502 | 7.1 | 9.8 | 77/78 | 60 | 21 |
| Moosvi 2018 [20] | NRCT | Cetrimide, chlorhexidine | 395 | 297 | 98 | 5.7 | 4 | 69.4/68.2 | 60 | 20 |
| Svensson Neufert 2021 [21] | NRCT | NR | 2425 | 265 | 2160 | 3.8 | 4 | 71/70 | 60 | 22 |
| Teurneau-Hermansson 2021 [22] | NRCT | NR | 4821 | 4317 | 504 | 1.2 | 2.4 | 67/68 | 60 | 22 |
| Svensson Neufert 2022 [23] | NRCT | Capecitabine | 6186 | 5706 | 480 | 1.7 | 2.5 | 67/68 | 36 | 22 |
Effect of rectal washout for local recurrence
In our study, a total of 7 articles reported data on the rectal washout versus no rectal washout for local recurrence, from which we conclude that RW for local recurrence is better than NRW. Our result gives a significant difference between the two groups (OR = 0.48, 95% CI: 0.40–0.57, p < 0.05) (Figures 2 A, B).
Figure 2
A – Forest plot of the comparison between RW and NRW for local recurrence which shows the total number of patients and the event shows the performed procedure. Asterisk shows RCT studies. Odds ratio indicates the estimated standardized mean difference and its corresponding confidence interval (OR = 0.48, 95% CI: 0.40–0.57). B – Funnel plot for local recurrence is shown for study bias
Effect of rectal washout for intraoperative washout
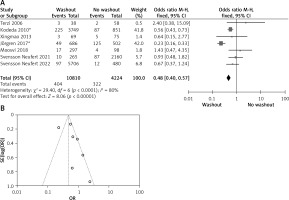
Six of the included articles provided data on intraoperative rectal washout for our final analysis. There were significant differences regarding intraoperative washout between RW and NRW groups (OR = 0.65, 95% CI: 0.53–0.80, p < 0.05) (Figures 3 A, B).
Figure 3
A – Forest plot of the comparison between RW and NRW for the intraoperative washout in which total shows the total number of patients and events shows the performed procedure. Asterisk shows the RCT studies. Odds ratio provides the estimated standardized mean difference and its confidence interval (OR = 0.65, 95% CI: 0.53–0.80). B – Funnel plot for intraoperative washout is shown for study bias
Effect of rectal washout on anastomosis
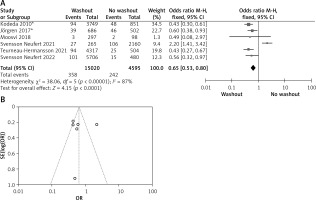
Statistical data for the evaluation of the RW group and NRW group on anastomotic leakage were obtainable from 3 studies including a total of 9546 treated patients comprising 8093 RW and 1453 NRW. Heterogeneity shows significant results but the overall result is not significant (OR = 0.97, 95% CI: 0.80–-1.18, p =0.76) (Figures 4 A, B).
Figure 4
A – Forest plot of the comparison between RW and NRW for anastomotic leakage in which total shows the total number of patients and events shows the performed procedure. Asterisk shows the RCT study. Odds ratio provides the estimated standardized mean difference and its corresponding confidence interval (OR = 0.97, 95% CI: 0.80–1.18). B – Funnel plot for anastomotic leakage is shown for study bias
Effect of rectal washout with neoadjuvant therapy
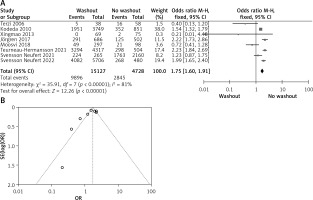
Statistical data for the evaluation of the RW group and NRW group on neoadjuvant therapy were obtainable from 8 different studies including a total number of treated patients of 19,855 in which the RW group included 15127 and NRW 4728. Our result indicates a statistically significant result but it favors the NRW group (OR = 1.75, 95% CI: 1.60–1.91, p < 0.05) (Figures 5 A, B).
Figure 5
A – Forest plot of the comparison between RW and NRW for the neoadjuvant therapy in which total shows the total number of patients and events shows the performed procedure. Asterisk shows the RCT studies. Odds ratio provides the estimated standardized mean difference and its associated confidence interval (OR = 1.75, 95% CI: 1.60–1.91). B – Funnel plot for neoadjuvant therapy is shown for study bias
Effect of rectal washout with adjuvant therapy
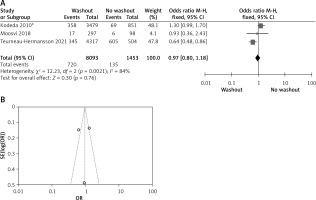
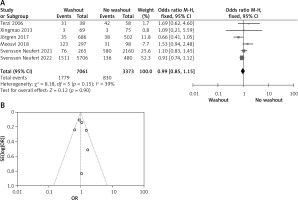
Statistical data for the evaluation of the RW group and NRW group on adjuvant therapy were obtainable from 6 different studies including a total number of treated patients of 10,434 in which the RW group included 7,061 and NRW 3,373. Our result indicates no statistically significant difference between the two groups (OR = 0.99, 95% CI: 0.85–1.15, p = 0.90) (Figures 6 A, B).
Figure 6
A – Forest plot of the comparison between RW and NRW for the adjuvant therapy in which total shows the total number of patients and events shows the performed procedure. Asterisk shows the RCT studies. Odds ratio provides the estimated standardized mean difference and its associated confidence interval (OR = 0.99, 95% CI: 0.85–1.15). B – Funnel plot for adjuvant therapy is shown for study bias
Effect of rectal washout with radial resection margin
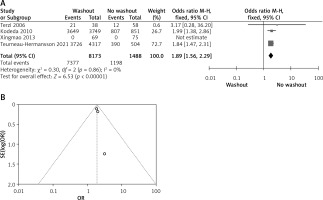
Statistical data for the evaluation of the RW group and NRW group on radial resection margin were obtainable from 4 different studies including a total number of treated patients of 9,661 in which the RW group included 8,173 and NRW 1,488. Overall result shows statistically significant result between the two groups (OR = 1.89, 95% CI: 1.56–2.29, p < 0.01) (Figures 7 A, B).
Figure 7
A – Forest plot of the comparison between RW and NRW for the radial resection margin in which total shows the total number of patients and events shows the performed procedure. Asterisk shows the RCT studies. Odds ratio provides the estimated standardized mean difference and its corresponding confidence interval (OR = 1.89, 95% CI: 1.56–2.29). B – Funnel plot for radial resection margin is shown for study bias
Subgroup analysis includes RCT studies
Effect of washout in RCT on local recurrence
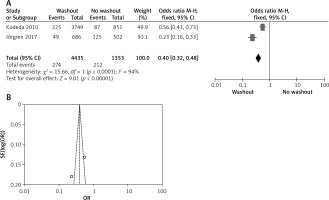
Two out of six included articles are RCT that provided data on local recurrence rectal washout. There were statistically significant differences observed in the local recurrence washout between the RW and NRW groups (OR = 0.40, 95% CI: 0.32–0.48, p < 0.05) (Figures 8 A, B).
Figure 8
A – Forest plot of the comparison between RW and NRW for the local recurrence in which total shows the total number of patients and events shows the performed procedure. Odds ratio gives the estimated standardized mean difference and its corresponding confidence interval (OR = 0.40, 95% CI: 0.32–0.48). B – Funnel plot for radial resection margin is shown for study bias
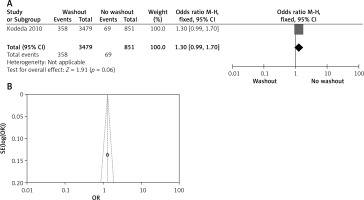
Effect of washout in RCT on intraoperative washout
Two out of six included articles are RCT that provided data on intraoperative rectal washout. There were significant differences regarding intraoperative washout between RW and NRW groups (OR = 0.50, 95% CI: 0.38–0.66, p < 0.05) (Figures 9 A, B).
Figure 9
A – Forest plot of the comparison between RW and NRW for the intraoperative washout in which total shows the total number of patients and events shows the performed procedure. Odds ratio gives the estimated standardized mean difference and its associated confidence interval (OR = 0.50, 95% CI: 0.38–0.66). B – Funnel plot for radial resection margin is shown for study bias
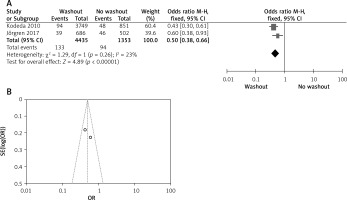
Effect of washout in RCT on neoadjuvant therapy
Two out of six included articles are RCT that provided data on neoadjuvant therapy rectal washout. There were significant differences regarding neoadjuvant therapy washout between RW and NRW groups (OR = 1.70, 95% CI: 1.49–1.93, p < 0.05) (Figures 10 A, B).
Figure 10
A – Forest plot of the comparisons between RW and NRW for the neoadjuvant therapy in which total shows the total number of patients and events shows the performed procedure. Odds ratio gives the estimated standardized mean difference and its corresponding confidence interval (OR = 1.70, 95% CI: 1.49–1.93). B – Funnel plot for radial resection margin is shown for study bias
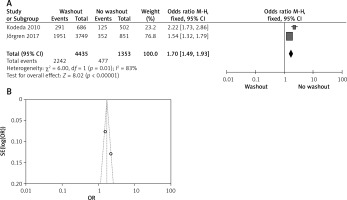
Effect of washout in RCT on adjuvant therapy
One out of six included articles are RCT that provided data on adjuvant therapy rectal washout. There were no statistically significant disparities observed in adjuvant therapy washout between the RW and NRW groups (OR = 0.66, 95% CI: 0.41–-1.05, p > 0.05) (Figures 11 A, B).
Figure 11
A – Forest plot of the comparison between RW and NRW for the adjuvant therapy in which total shows the total number of patients and events shows the performed procedure. Odds ratio gives the estimated standardized mean difference and its associated confidence interval (OR = 0.66, 95% CI: 0.41–1.05). B – Funnel plot for radial resection margin is shown for study bias
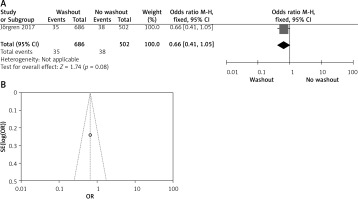
Effect of washout in RCT on anastomosis leakage
One out of six included articles are RCT that provided data on anastomosis leakage rectal washout. There were no significant differences regarding anastomosis leakage washout between RW and NRW groups (OR = 1.30, 95% CI: 0.99–1.70, p > 0.05) (Figures 12 A, B).
Figure 12
A – Forest plot of the comparison between RW and NRW for the anastomosis leakage in which total shows the total number of patients and events shows the performed procedure. Odds ratio gives the estimated standardized mean difference and its corresponding confidence interval (OR = 1.30, 95% CI: 0.99–1.70). B – Funnel plot for radial resection margin is shown for study bias
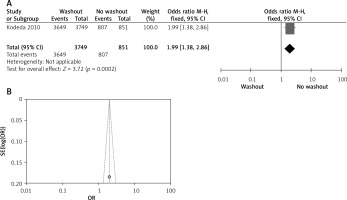
Effect of washout in RCT with radial resection margin
One out of six included articles are RCT that provided data on radial resection margin rectal washout. There were significant differences observed regarding radial resection margin washout between RW and NRW groups (OR = 1.99, 95% CI: 1.38–2.86, p < 0.05) (Figures 13 A, B).
Figure 13
A – Forest plot of the comparison between RW and NRW for the radial resection margin in which total shows the total number of patients and events shows the performed procedure. Odds ratio gives the estimated standardized mean difference and its associated confidence interval (OR = 1.99, 95% CI: 1.38–2.86). B – Funnel plot for radial resection margin is shown for study bias
Discussion
The goal of this study was to assess RW efficacy and safety in rectal cancer patients. There were 19,855 participants in the eight studies, with 15,127 RW patients and 4728 NRW patients in the RW group [16–23]. The findings imply that rectal washout reduces local recurrence and the detection of free cancer cells in patients undergoing anterior resection for rectal cancer. Rectal washout was linked to better results for patients undergoing surgery for distal colonic or rectal cancer, according to the study’s main finding.
Rectal cancer local recurrence is one of the biggest challenges for surgeons since it affects the prognosis of the disease. Patients who experience local recurrence live shorter lives and with lower quality of life. Numerous factors, including the presence of a solid tumor, the stage of the tumor, whether or not a radical margin was performed, the response to adjuvant therapy, and others, have been linked to local recurrence. The process underlying local recurrences has been hypothesized to involve the implantation of exfoliated malignant cells. Although our results are similar to those of earlier meta-analyses [11–13], in order to achieve better results, we examined more patients in our study.
Some surgeons have used intra-operative rectal washout in clinical practice. Although there are a few case reports describing major adverse effects following the application of frequently used solutions such as cetrimide and chlorhexidine, most surgeons believe that rectal washout is safe [24, 25]. According to these findings, distal colon washout lowers septic morbidity after civilian rectal injuries [26].
Recently, a substantially greater number of patients have undergone neoadjuvant treatment, whereas earlier meta-analyses rarely described adjuvant therapy. According to a new study, compared to historical controls treated with chemoradiotherapy, total mesorectal excision (TME), and postoperative chemotherapy, organ preservation is possible in half of rectal cancer patients treated with comprehensive neoadjuvant therapy, without appearing to have an adverse effect on survival. For patients with locally advanced rectal cancer, short-term radiation combined with preoperative chemotherapy and surgery was effective and had a manageable level of toxicity [27, 28]. Neoadjuvant therapy may favor no rectal washout in our study since a high proportion of patients were selected for this group in previous clinical studies; therefore, an RCT is necessary to rule out this discrimination.
The diminished LR risk is the primary justification for conducting RW. Conflicting evidence exists on the effect of RW on LR. A sizable study on sphincter-preserving complete response to chemoradiotherapy (SCRCR), along with current systematic reviews and meta-analyses, consistently demonstrate that RW significantly reduces LR [29–31]. Power calculations demonstrate that a sample size of at least 1400 patients and a follow-up period of 5 years are required for an RCT, which is a barrier to its execution. Furthermore, the majority of European colorectal surgeons adopted the TME surgery approach at the time and were persuaded of the significance of RW. Therefore, some authors think it would be unethical to conduct an RCT [17].
Aside from anastomotic recurrence, a specific type of local recurrence, implantation of free viable cancer cells, may be responsible for various types of local recurrence. According to McGregor et al., cancer cells remain alive and free in the residual rectal lumen and spread into the anastomosis [4]. Furthermore, it has been demonstrated that viable rectal cancer cells can pass through a watertight anastomosis and perhaps result in local recurrences [32, 33].
Our study gave a positive result of radial resection margin toward NRW and it shows a statistically significant result. The circumferential resection margin (CRM), also known as the radial margin, is an essential factor in rectal cancer surgery and can be used to predict which patients would benefit from the procedure. However, a positive microscopic radial margin was associated with a greater likelihood of distant recurrence [34]. Other studies have confirmed the importance of radial resection margin involvement in terms of local recurrence and prognosis [35–37].
In this meta-analysis, we propose that the RW procedure offers a significantly higher quality of life after local recurrence compared to NRW. This finding suggests that RW may be collectively recommended on a broader scale with statistical significance. The effectiveness of our meta-analysis is largely due to two things. First, we looked for studies that minimize the confounders that are a natural part of observational studies. Furthermore, this approach was employed on a specific population to assist the surgeon in determining whether an RW or NRW is superior.