Summary
Percutaneous left atrial appendage closure (LAAC) is an alternative option to permanent oral anticoagulation (OAC) therapy for stroke prevention in patients with non-valvular atrial fibrillation and who are not a good candidate to lifelong OAC. However, LAAC devices are made of artificial material and initially after implantation they have potential to initiate thrombus formation on the atrial surface of the device. Despite the suggested post-procedural regimen we still observe device-related thrombus (DRT) during a follow-up visit, which becomes an even bigger issue in patients with initially high bleeding risk. In our study we showed that this is not a common complication after LAAC procedure with no further thromboembolic events in our population. Moreover, we were able to demonstrate several risk factors for DRT formation which can be helpful in identification of patients with a higher risk of DRT.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and its most severe complication is ischemic stroke. Oral anticoagulation (OAC) therapy reduces the risk of stroke in patients with AF but simultaneously it increases the risk of bleeding complications [1]. Percutaneous left atrial appendage closure (LAAC) is an alternative option to permanent OAC for stroke prevention in patients with non-valvular AF and who are not a good candidate to lifelong OAC [2]. The main aim of the procedure is to completely seal the left atrial appendage (LAA) which is the main spot where the blood clots originate [3]. Thereby we eliminate the main source of emboli, thus we also eliminate the indication to OAC therapy [1]. The main evidence supporting LAAC procedure comes from the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) and PREVAIL (Watchman LAA Closure Device in Patients with Atrial Fibrillation Versus Long Term Warfarin Therapy) randomized trials [4, 5]. Large observational studies also support the safety and efficacy of percutaneous LAAC procedure [6–8]. However, LAAC devices are made of artificial material and until its fully endothelialization they have potential to initiate thrombus formation on the atrial surface of the device, by what they promote subsequent systemic embolization [9]. The device-related thrombus (DRT) has been observed in 0% to 17.6% of the patients and it occurs mainly early after the procedure [10]. We still do not know the clinical importance of DRT and whether it is associated with more frequent episodes of ischemic stroke [11]. Moreover, an optimal postprocedural antithrombotic regimen remains unknown. In patients with a high bleeding risk who are not suitable to OAC therapy current guidelines recommend initiating dual antiplatelet therapy (DAPT) after successful LAAC procedure with Watchman device to prevent thrombus formation [2]. However, despite this regimen we still observe DRT during follow-up visits, which becomes an even bigger issue in patients with initially high bleeding risk.
In our department we showed that DAPT seems to be safe and efficient after successful LAAC, while there is still a risk for DRT formation [12].
Aim
Thus, we decided to conduct this study to characterize the incidence and predictor of DRT after LAAC procedure with first-generation Watchman 2.5 device.
Material and methods
Study population
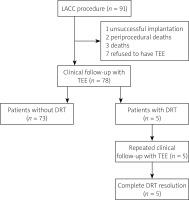
The flow chart of this study is presented in Figure 1. Retrospectively we have reviewed 91 consecutive patients who were qualified to LAAC procedure with first-generation Watchman 2.5 device from March 2015 to September 2019 in the single cardiological department (First Department of Cardiology, University Clinical Center, Warsaw, Poland). All patients were diagnosed with non-valvular AF and were at high thromboembolic risk assessed in CHA2DS2-VASc score. Moreover, they also fulfilled at least one of the following inclusion criteria: contraindication for oral anticoagulation, history of the bleeding complication while using oral anticoagulation, inability to maintain the INR level within therapeutic range, history of a stroke while using oral anticoagulation. All patients did not meet any of the following exclusion criteria: presence of the thrombus in left atrial appendage, too small or too big left atrial appendage in qualifying transesophageal echocardiography (TEE), lack of patient’s consent. All operators who performed the procedures attended an appropriate training and certification program to minimize the risk of the procedure. In our analysis we have included all patients (n = 78) who had clinical follow-up visits with TEE.
LAAC procedure and postimplantation therapy regimen
The procedure was performed under general anesthesia with TEE and fluoroscopic guidance. Left atrial access was obtained through a transseptal puncture at the inferior and posterior part of the fossa. Unfractionated heparin was administered to achieve activated clotting time > 250 s. Several views from 0° to 135° were used initially to assess the LAA to choose the most suitable size of the device. The final deployment of the device was done under TEE and fluoroscopic guidance. After device implantation, a stability test was performed before its final release. Contrast angiography and color-Doppler in TEE were used to eliminate the peri-device leaks. If needed, recapturing and reimplantation were performed. All patients were treated with DAPT (aspirin 75 mg and clopidogrel 75 mg once a day) or with single antiplatelet therapy (SAPT) (aspirin 75 mg or clopidogrel 75 mg once a day). A decision on the therapy regimen was made according to the implanting physician’s judgement and was continued until control examination. The decision was based on patients’ medical history, especially history of bleeding or ischemic event, bleeding and thromboembolic risk scores, patients’ comorbidities, and procedure outcomes.
Follow-up
The follow-up visit was planned to be performed approximately 3 months after successful LAAC procedure. During the follow-up visit clinical data were obtained and all patients had TEE examination to evaluate the device position and the presence of DRT. TEE imaging was performed in 4 planes (0°, 45°, 90°, and 135°) as recommended and was evaluated by an experienced specialist.
DRT was defined as an echo density on the left atrial aspect of the device: (1) not explained by the imaging artifact; (2) inconsistency with normal healing/device incorporation; (3) visible in multiple TEE planes; (4) in contact with the Watchman device; and (5) exhibiting independent motion [13]. In all patients during TEE, color-flow Doppler was used to detect a peri-device leak and measurements were taken in different planes to identify the maximal jet dimension. Non-significant peri-device leak was defined as a jet into the LAA < 5 mm. We also evaluate the depth of the implanted Watchman device. We measured the distance between the left atrial appendage occluder and the ridge of the upper left pulmonary vein, so the distance of the uncovered rim was measured.
Statistical analysis
Categorical variables, presented as frequencies/counts and percentages, were compared using χ2 test of Fisher’s exact test, as appropriate. Continuous variables, presented as mean with standard deviation (SD) if normally distributed or by median and interquartile range (IQR) if not, were compared using a Student’s t test or a Mann-Whitney U test, as appropriate. Univariate analysis was performed to evaluate the predictors of DRT in the overall cohort. Results are presented as odds ratio (OR) with 95% confidence intervals (CI). P-value of < 0.05 was considered statistically significant. All statistical analyses were conducted with SPSS Statistics, version 22 (IBM SPSS Statistics, New York, USA).
Results
Incidence of DRT
Ninety-one patients underwent the procedure of LAAC in our clinic in the defined period. A clinical follow-up with imaging was available in 78 (85.7%) patients in our study. Thirteen patients were excluded from further analysis because of one unsuccessful implantation, 2 periprocedural deaths, 3 patients died during follow-up (2 non-cardiovascular death and 1 caused by exacerbation of heart failure), and 7 patients refused to have TEE. The median (IQR) follow-up time for the entire group was 68 (51.75–82.25) days. Among these patients, the diagnosis of DRT was made in 6.4% (5/78) after the median time of 76 (68–302) days. All patients with DRT received DAPT after the procedure.
Patients with and without DRT
Comparison of clinical baseline characteristics of patients with and without DRT is presented in Table I. No significant differences were noted regarding gender (male: 60% versus 52.1%, p = 1), but it was observed that patients with DRT were significantly younger than patients without DRT with a mean (SD) age of 67.4 years (7) versus 75 years (8.3) (p = 0.045). The median thromboembolic risk assessed with CHA2DS2-VASc score were similar in both groups (4 (4.0–5.0) versus 5 (4.0–6.0), p = 0.62). Furthermore, the median bleeding risk assessed with HAS BLED was also similar in both groups (3 (3.0–4.0) versus 3 (3.0–4.0), p = 0.49), but we have observed a trend of lower bleeding risk assessed with ATRIA in patients with DRT than in patients without, but it did not meet criteria for statistical significance (2 (1.5–3.5) versus 4 (2.0–7.0), p = 0.07). Permanent AF had a tendency to be more often diagnosed in patients with DRT than the other group, but it was also not a statistically significant difference (80% versus 34.2%, p = 0.06). The prevalence of hypertension (80% versus 87.8%, p = 0.51), diabetes (20% versus 37%, p = 0.65), and history of prior thromboembolism (40% versus 24.7%, p = 0.6) did not differ significantly between both groups. The glomerular filtration rate (GFR) was similar in both groups (64 ml/min/1.73 m2 (39.5–77.0) versus 52.5 ml/min/1.73 m2 (38.5–76.0), p = 0.57), and platelet count was not significantly different in studied populations (162 × 109/l (150.0–196.5) versus 200 × 109/l (164.0–248.5), p = 0.14).
Table I
Baseline characteristics
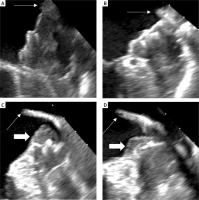
All procedural and echocardiographic characteristics are shown in Table II. In transthoracic echocardiography (TTE) evaluation, patients with DRT had significantly lower ejection fraction (EF) (40% (23.5–45.5) versus 55% (48.0–60.0), p = 0.005). Regarding preprocedural TEE parameters, patients with DRT showed a significantly larger LAA diameter assessed in 900 plane (22.4 mm (3.2) versus 19 mm (2.7), p = 0.02), and in 1350 plane (23 mm (3.2) versus 19.9 mm (2.5), p = 0.03). We also observed a significantly larger depth of LAA (35 mm (29.5–41.0) versus 29 mm (25.5–31.0), p = 0.03), and significantly lower emptying velocity of the LAA (25 cm/s (17.5–27.0) versus 53 cm/s (26.5–78.0), p = 0.009). Both groups did not differ significantly with respect to the LAA diameter in any other plane and to the dimension of the left atrium (51 mm (46.0–52.0) versus 44 mm (40.0–52.0), p = 0.24). The rate of spontaneous echo contrast (SEC) in TEE and presence of non-significant peri-device leak was almost equal in both groups (Table II). In follow-up TEE the device compression was comparable in both groups (16.67% (14.07–18.33) versus 16.67% (14.81–20.83), p = 0.42), but the depth of the device implantation, measured as a distance between the surface of the device and the edge of the pulmonary ridge, was significantly greater in patients with DRT (18 mm (14.0–20.5) versus 8 mm (5.0–11.0), p < 0.001) (Figure 2).
Table II
Procedural and echocardiographic characteristics
| Variable | All (n = 78) | DRT (n = 5) | No DRT (n = 73) | P-value |
|---|---|---|---|---|
| EF, % | 55 (45.0–60.0) | 40 (23.5–45.5) | 55 (48.0–60.0) | 0.005 |
| LA dimension in TTE [mm] | 44 (41.0–52.0) | 51 (46.0–52.0) | 44 (40.0–52.0) | 0.24 |
| Emptying velocity of LAA [cm/s] | 52.5 (25.75–73.75) | 25 (17.5–27.0) | 53 (26.5–78.0) | 0.009 |
| LAA depth [mm] | 29 (26.0–31.25) | 35 (29.5–41.0) | 29 (25.5–31.0) | 0.03 |
| Dimension in 0° [mm] | 19.8 (3.1) | 22 (2.5) | 19.7 (3.1) | 0.08 |
| Dimension in 45° [mm] | 19.4 (2.4) | 21.2 (1.8) | 19.3 (2.4) | 0.06 |
| Dimension in 90° [mm] | 19.2 (2.9) | 22.4 (3.2) | 19 (2.7) | 0.02 |
| Dimension in 135° [mm] | 20.1 (2.6) | 23 (3.2) | 19.9 (2.5) | 0.03 |
| SEC | 15 (19.2%) | 1 (20%) | 14 (19.2%) | 1 |
| Device size [mm] | 27 (24.0–27.75) | 30 (25.5–30.0) | 27 (24.0–27.0) | 0.11 |
| Compression, % | 16.67 (14.81–20.21) | 16.67 (14.07–18.33) | 16.67 (14.81–20.83) | 0.42 |
| Depth of implantation [mm]* | 9 (5.0–12.0) | 18 (14.0–20.5) | 8 (5.0–11.0) | < 0.001 |
| Non-significant peri-device leak** | 16 (20.5%) | 1 (20%) | 15 (20.5%) | 1 |
Unless indicated otherwise, data are given as the mean (standard deviation), median (interquartile range) or as n (%). EF – ejection fraction, LA – left atrium, LAA – left atrium appendage, SEC – spontaneous echo contrast, TTE – transthoracic echocardiography.
Figure 2
Transesophageal echocardiography follow-up imaging after left atrial appendage closure with the Watchman device. A, B – optimal Watchman 30 mm device placement without deep implantation and device-related thrombus (DRT). C, D – deep implantation of Watchman 27 mm device with DRT indicated with a thick arrow. Thin arrows indicate the pulmonary ridge
Univariate regression analysis was performed to evaluate predictors of DRT (Table III). This analysis showed that the deeper device implantation (OR = 1.25, 95% CI: 1.04–1.51, p = 0.02) and the bigger dimension of LAA in 900 plane (OR = 1.47, 95% CI: 1.0–2.1, p = 0.02), the higher the risk of DRT development. Moreover, it has been shown that the lower the emptying velocity of the LAA (OR = 0.94, 95% CI: 0.8–0.99, p = 0.046), and EF (OR = 0.89, 95% CI: 0.81–0.96, p = 0.005), the higher the risk of DRT.
Table III
Univariate analysis evaluating most important predictors of DRT.
During the follow-up period only one stroke was diagnosed, and no DRT was found in this patient.
Follow-up and management after DRT diagnosis
All patients diagnosed with DRT were prescribed to have OAC therapy. Four of them had low molecular weighted heparin (LMWH) at a therapeutic dose adjusted to the kidney function and 1 patient was treated with warfarin. The vitamin K antagonist (VKA) was prescribed because the patient refused to have subcutaneous injections with LMWH and refused to have new oral anticoagulants (NOAC) because of their high price. All patients have repeated clinical follow-up with TEE after a median time of 63 (54.5–99.0) days. Resolution of DRT was confirmed by follow-up TEE in all patients.
In all patients with a confirmed thrombus at first follow-up imaging, there were no reports of ischemic complications such as a stroke, TIA, or peripheral embolism during the period following DRT detection. Moreover, no one experienced any bleeding complication after the change of the anticoagulation regimen.
Discussion
Device-related thrombus remains one of the most concerning complications after the successful LAAC procedure with Watchman device which can be confirmed by control imaging methods [14]. Initially, the presence of DRT varied from 0% to even up to 17.6% of the cases [10, 15], while currently available data show that the incidence of DRT has dropped, and varies from 2 to 4% [16]. Thus, we may suspect that clinical and echocardiographic, as well as procedure- and device-related factors contribute to the formation of DRT.
In our single center study, thrombus formation following LAAC with Watchman device was observed in 6.4% of the patients after the median time of 76 (68–302) days. In the sub-analysis of the EWOLUTION registry, Sedaghat et al. showed that more than 91% of the DRTs were detected relatively early after the procedure after the median of 54 days [17]. Moreover, one of the largest trials regarding DRT has shown that almost 64% of DRT diagnoses were made within the first 180 days after the procedure [18]. Our rate of thrombus incidents with this nitinol cage device is slightly higher than of these observed in PROTECT AF with an incidence of 5.7% [13]. In the ASAP study, 6 out of 142 patients with a successfully implanted Watchman device have developed DRT, with a rate of 4.2% [19]. Dukkipati et al. evaluated the incidence of DRT among the patients who underwent Watchman device implantation as a part of PROTECT AF or PREVAIL randomized trials, as well as CAP or CAP2 registries and revealed that the rate of DRT was 3.74% [9]. However, 252 patients included into the PREVAIL trial were not assessed for DRT, thus the incidence of DRT in this population remains unknown [5]. Our study reflects data obtained in everyday clinical practice where we encounter patients who probably have more comorbidities, are older and have higher CHA2DS2-VASc score than these included in randomized clinical trials or big registries.
Optimal post-procedural treatment after LAAC for the occurrence of DRT is still controversial. Previously mentioned randomized trials PROTECT AF and PREVAIL used warfarin for the first 45 days after implantation but these studies included patients who did not have absolute contraindications to OAC. The largest multicenter, prospective registry of Watchman device implantation, EWOLUTION registry, assessed patients with a variable post implantation treatment regimen. Single or dual antiplatelet therapy was used in 67% of patients, direct oral anticoagulants in 11%, VKA in 16%, and 6% did not receive any form of antithrombotic treatment [20]. Comparing all these different post-procedure treatment regimens, no significant differences were noted in the rate of DRT frequency and in the overall event rate. Bergmann et al. in a subsequent analysis of a group of patients from the EWOLUTION trial, who were treated with DAPT, revealed that the rate of DRT was 4.0% without any adverse consequences using this antithrombotic regimen at 12 months [21]. However, in 2019, Søndergaard et al. performed cumulative analysis including several studies of Watchman device and reported that there is an increased risk of DRT in patients without post-procedure OAC [22]. In our study, all patients in whom DRT was detected were on DAPT. It is worth underlying that thrombus formation in patients treated with DAPT may be due to their clopidogrel resistance [23]. However, there are some studies that have proven safety and efficacy of the single antiplatelet therapy without any negative influence on ischemic events [24, 25].
In addition, post-procedural clinical, procedural and echocardiography-related factors have a big impact of the occurrence of DRT. Patients in whom DRTs were diagnosed had lower EF, deeper LAA, and a bigger dimension of LAA especially when assessed in 90° and 135° planes. That may indicate a greater LA dimension in a group with DRT, which would be in line with results from other larger studies, but our results did not show statistical significance in terms of the dimension of LA. Additionally, we have proved that lower emptying velocity of LAA, which reflects impaired left atrial function, is strongly connected with further DRT formation. Surprisingly, it is not associated with SEC detection, which is a known risk factor for thrombus formation inside of LAA [26]. SEC was observed with a similar rate in our population (20% versus 19.2%). Referring to the impairment of LA function, it is proved that remodeling leading to atrial fibrosis is a known risk factor for a thromboembolic complication [27]. The study of Daccarett et al. revealed bigger remodeling of the LA detected in cardiac magnetic resonance, especially in patients with permanent AF [28]. In our patients with DRT, we saw a greater prevalence of permanent AF rather than paroxysmal, but the difference did not reach statistical significance. Finally, we were able to demonstrate that the deep Watchman device implantation is associated with a higher risk of DRT. In comparison to DRT-free patients, the distance between the pulmonary ridge and the device was significantly greater in the DRT population. This observation of deep implantation has been described previously claiming that residual LAA has adequate blood stasis promoting thrombus formation [29]. Simard et al. confirmed this finding in their larger, multicenter study and they claimed that due to varying anatomies, anatomical landmarks, and different device types, the depth measurement can be challenging [18]. However, it can be helpful in standardizing the evaluation of the occluder as it relates to prediction of DRT.
In comparison to other studies, the presence of DRT in our group was not linked with an increasing risk of thromboembolic complications, which goes along with results of the large EWOLUTION registry [17]. We have not observed any stroke or TIA in patients with diagnosed DRT. Dukkipati et al. in the previously mentioned analysis of 4 prospective FDA approved trials, have reported an increased rate of ischemic stroke and systemic embolism incidence [9]. Other studies also concluded that DRT is strongly associated with a higher risk of stroke and TIA during follow-up [11, 18]. In our population we have not observed many DRTs and all of them were diagnosed after the median time of 76 days after the procedure, so the appropriate treatment was initiated relatively quickly. One patient received VKA with warfarin and the rest of the patients received LMWH after DRT diagnosis. This antithrombotic therapy was continued until next follow-up imaging. Sedaghat et al. showed that different treatment regimens, including NOAC, VKA, or heparin have similar resolution rates [27]. Thus, considering high bleeding risk in our population our first choice was LMWH. Finally, we achieved complete resolution of DRT, with no subsequent bleeding complications associated with treatment change.
Until the present day the TEE has remained gold standard in DRT detection. Nevertheless, cardiac computed tomography (CT) has also good clinical value for detection of DRT, and it is a good diagnostic method in patients who cannot tolerate or who have contraindications to TEE. Korsholm et al. demonstrated that cardiac CT seems to be equally good as TEE for DRT diagnosis [30]. It has also the ability to detect the presence of low-grade hypoattenuated thickening (HAT) on the device, but no thromboembolic events were present in a group diagnosed with low-grade HAT. However, performing a cardiac CT involves exposure to radiation and the contrast agent. Furthermore, patients with a rapid, irregular heart rate are not a good candidate for CT due to lower scan resolution.
On the basis of our study as well as previous ones we may conclude that DRT remains an issue after first-generation Watchman device implantation and this problem needs further evaluation. Furthermore, a study evaluating the optimal post-procedural drug regimen with regard to DRT risk factors is needed.
Limitations
These results are based on the retrospective registry with all limitations associated with this study design. Further randomized trials are needed to confirm study findings. The population was relatively small. The follow-up imaging was not available in all cases with Watchman implantation and the follow-up time was limited. Therefore the actual rate of DRT may be overestimated or underestimated. Finally, DRT was diagnosed by the physician not blind to primary implantation results, without a central evaluation of images in a core-lab. Results of the study included first-generation Watchman device for LAAC procedure, so our outcomes not necessarily concern other devices, such as last generation of Watchman Flex device or Amulet device.
Conclusions
The DRT after Watchman device implantation remains a rare complication and, in our population, it was not associated with an increased risk for ischemic stroke or any other thromboembolic event. We were able to demonstrate several risk factors for DRT formation which can be helpful in identification of patients with a higher risk of DRT. In general, we do not have any influence on most of the risk factors like age, and LAA dimension, but to minimize the risk of DRT, the deep implantation should be avoided if possible.