Introduction
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are well known and widely used liver function tests. The increased activity of these enzymes is usually interpreted as the laboratory parameter of liver cell damage and followed by a diagnostic work-up to determine the underlying disease. In a small number of patients the differential diagnosis does not show any other abnormalities either in the laboratory or in imaging studies. The reason for increased ALT/AST in these patients is the presence of macroenzymes that cause a false increase of ALT/AST activity in the standard laboratory tests [1–3].
Macroenzymes are big particles resulting from polymerisation or connection of ALT/AST or pancreatic amylase with serum immunoglobulins or lipoproteins. They may be present in healthy subjects. Macroenzymes can emerge in patients with chronic hepatitis C, autoimmune diseases including rheumatoid arthritis, lupus erythematosus, ulcerative colitis, coeliac disease and some neoplastic disorders. MacroAST is most frequently detected. There are two AST isoenzymes: one located in the cytoplasm and the other one located in mitochondria. They are coded with GOT1 and GOT2 located on chromosomes 10q24 and 16q12. The isoenzyme that forms macroAST is usually derived from cytosol [4].
Detection of macroenzymes requires two simultaneous tests on the same blood sample. The first one is a standard laboratory test and the second test is done with the addition of polyethylene glycol (PEG), which releases a standard enzyme from the connection with big particles.
Method of detection of macroenzymes ALT and AST
Aspartate aminotransferase and ALT macroenzymes can be determined by PEG precipitation using a 25% PEG solution. The PEG solution is prepared by adding polyethylene glycol PEG 6000 (for synthesis) (807491 Sigma-Aldrich) to 0.9% sodium chloride (NaCl). The PEG solution is stored at 4°C. To test for the presence of the macroenzyme, 150 µl of patient serum is pipetted into a tube, then another 150 µl of PEG solution is added and vortexed for 10 s. The mixture is incubated at 37°C for 10 minutes, then centrifuged at 3000 rpm for 20 minutes at room temperature. For the blank, 150 µl of deionized water is added instead of PEG solution. The sample and blank are simultaneously treated in the same way. The PEG precipitated activity (PPA) is calculated by measuring the level of aminotransferases in the probe supernatants and using the formula: %PPA = [(blank activity – PEG activity)/blank activity] × 100. The cut-off values for PPA according to Davidson and Watson [5] are 74% PPA for macroALT and 55% PPA for macroAST.
The confirmation of the presence of macroAST and macroALT can be done with electrophoretic separation because macroenzymes have characteristic mobility due to the electrical charge of the subunits.
Serum samples of 5 µl and 5 µl of PEG supernatant are applied to agarose gel (8%, pH 8.40 ±0.05), which prior to that is mixed with 5 µl of activation solution containing β-mercaptoethanol and incubated for 10 minutes at about 25°C. The agarose gel is placed in a migration chamber. After migration (50 W, 1.5 h, 24°C), the reaction is stopped and macroAST and macroALT are visualized based on the reaction to a chromogenic compound. The gels are then washed, dried by heating and placed on a scanner. The scanned images are visually checked for any separation errors, which if detected are manually corrected using the appropriate software tools [4, 6].
Case description
We describe a 28-year-old woman with cystic fibrosis (CF) and rheumatoid arthritis. The patient had a CF related decreased pulmonary function but did not present signs or symptoms of CF related liver disease. The patient had normal bilirubin, normal ALT/AST and γ-glutamyltranspeptidase (GGTP) activity. The patient had slightly increased liver echogenicity on ultrasound examination but without focal lesions, cholelithiasis, biliary tract abnormalities or decreased portal vein flow. Liver elastography was normal (4.8 kPa).
The patient was treated with a low dose of prednisone due to rheumatoid arthritis and in May 2022 started cystic fibrosis transmembrane regulator (CFTR) modulator treatment receiving elexacaftor/tezacaftor/ivacaftor + ivacaftor therapy, showing rapid improvement of the general condition and pulmonary function (Fig. 1). Steroids were discontinued in Aug 2022 and the patient continued CFTR modulator treatment. Blood tests done in September 2022 showed an increase of ALT and AST activity without any clinical signs or symptoms of liver disease and without other laboratory abnormalities. Liver function tests were repeated every few weeks. The results remained stable and abnormal with a peak in November 2022. At that moment elexacaftor/tezacaftor/ivacaftor and ivacaftor were stopped and the patient was referred for hepatology consultation. The patient was screened for possible concomitant liver disease. These tests were negative for viral liver disease (HBV, HCV, EBV, CMV), autoimmune hepatitis and metabolic diseases (Wilson’s disease and α1-antiprotease deficiency). The patient did not present any signs or symptoms of liver dysfunction other than increased aminotransferases and ALT/AST normalized; thus elexacaftor/tezacaftor/ivacaftor + ivacaftor treatment was reintroduced with an immediate rise of ALT/AST activity (Fig. 1).
Fig. 1
Fluctuation of AST/ALT activity during elexacaftor/tezacaftor/ivacaftor + ivacaftor therapy and fluctuation of macroenzymes after reintroduction of CFTR modulator treatment
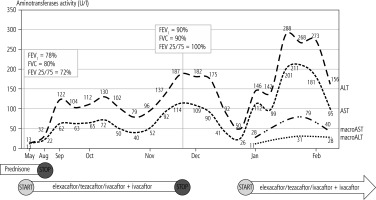
The patient’s blood was tested for the presence of macroenzymes and the result was positive. The patient is continuing elexacaftor/tezacaftor/ivacaftor + ivacaftor therapy and is double tested for ALT/AST activity. A standard laboratory test showed a marked increase of ALT/AST activity while a test with PEG showed normal values (Table 1). The presence of macroenzymes was also confirmed by an electrophoresis test.
Table 1
ALT/AST results in a standard laboratory test and in a test with PEG. Macroenzymes are detected if PEG related activity is greater than 55% for AST and 74% for ALT
| Date | Test | Standard test (U/l) | Test with PEG (U/l) | % of activity related to PEG |
|---|---|---|---|---|
| 6.01.2023 | ALT | 146 | 11 | 92% |
| AST | 112 | 28 | 75% | |
| 2.02.2023 | ALT | 268 | 31 | 88% |
| AST | 211 | 79 | 63% | |
| 25.02.2023 | ALT | 156 | 28 | 82% |
| AST | 95 | 40 | 58% |
Discussion
Alanine aminotransferase and AST are intracellular enzymes present in hepatocytes. The increase of serum activity of these enzymes is usually a result of cell damage; thus ALT and AST are used as markers of liver disease. Monitoring of ALT/AST activity is a usual practice during treatment with potentially hepatotoxic medications. It is recommended to consider dose reduction if the results become abnormal and to discontinue potentially hepatotoxic medication when the ALT/AST activity is above 8 × the upper limit of normal (ULN) or if it is above 5 × ULN for more than two weeks or above 3 × ULN with increased bilirubin, prolonged prothrombin time or clinical symptoms of liver disease [7].
Approximately 30% of CF subjects have underlying cystic fibrosis related liver disease (CFLD) [8] and CFTR modulators are known to have the potential to increase the ALT activity. There were case reports on drug induced liver injury (DILI) during CFTR modulator therapy [9, 10]. CFTR modulators’ characteristics inform about the risk of increase of liver function test outputs during the therapy and recommend adjustment of the dose to the stage of the patient’s liver disease. However, in the long term observation CFTR modulators may have a beneficial effect on liver function [11] and it may improve markers of liver fibrosis [12]. It is speculated that CFTR modulator treatment may lower the rate of patients progressing to liver cirrhosis in comparison with no treatment or single UDCA therapy [13]. For that reason liver function monitoring is an important aspect of CFTR modulator therapy.
The patient presented above had marked improvement of pulmonary function and general condition during CFTR modulator therapy. When abnormal liver function was detected, the patient and her physician were afraid that the treatment would have to be discontinued and the positive therapeutic effect would be lost. Thus intensive actions recommended for DILI were undertaken, resulting in diagnosis of the presence of macroenzymes as the possible explanation for increased ALT/AST activity. Based on these results the patient is continuing CFTR modulator treatment under double testing for ALT/AST with standard and PEG tests. The presence of macroenzymes was confirmed at every occasion. The patient does not present any laboratory signs of liver disease other than increased ALT/AST and does not report any clinical symptoms that could be related to liver disease.
The presence of macroenzymes in blood can cause diagnostic confusion. MacroAST and macroamylases are most frequently reported but the presence of macroenzymes was documented for other enzymes including ALT, GGTP, alkaline phosphatase (ALP), lipase, lactate dehydrogenase (LDH) and creatinine phosphokinase (CPK). The presence of all macroenzymes may be detected by the PEG precipitation test and the PPA indexes were calculated for all of the enzymes listed above [14]. The PEG precipitation test was used in our patient and it showed the presence of both macroAST and macroALT on several occasions. The presence of macroenzymes was confirmed by the electrophoresis test; thus macroenzymes can explain the aminotransferase flare observed in our patient.
Before starting CFTR modulator treatment the patient described in this report had increased liver echogenicity but no other signs or symptoms of CFLD, so we think that it is unlikely that elevation of ALT/AST could have been related to the exacerbation of chronic liver disease. The other diseases that could raise ALT/AST activity were excluded according to ASL guidelines for the approach to suspected DILI episode [7]. The pattern of ALT/AST elevation in relation to CFTR modulator therapy with a decrease during CFTR discontinuation and increase with CFTR modulator therapy re-challenge may indicate DILI. However, our patient has continued CFTR modulator treatment without further liver function deterioration and all the time has been positive for macroALT/macroAST.
It is possible that our patient developed CFTR modulator therapy related DILI as the main reason for ALT/AST elevation. However, in that case the patient should be negative for macroenzymes. The other possible interpretations of our case are the coincidence of DILI with the presence of macroenzymes or triggering of macroenzyme formation by CFTR modulator therapy. In the latter situation one could expect that with the cessation of CFTR modulator therapy the level of macroenzymes will fall and will rise with CFTR modulator re-challenge, as occurred in our subject.
The reason for macroenzyme formation is not well understood and to our knowledge the problem has not been studied in CF patients in relation to CFTR modulator therapy. Further studies are needed to test whether macroenzymes may be responsible for ALT/AST flares in subjects with CF. The problem is important for CF subjects treated with CFTR modulators because ALT/AST flares are frequent reasons for CFTR modulator treatment discontinuation with loss of the therapeutic effect on pulmonary function.
The detection of macroenzymes in our CF subject allowed us to continue CFTR modulator therapy despite abnormal results of standard test detecting ALT/AST activity. On the other hand, macroenzyme detection does not exclude DILI; thus our patient remains under careful hepatology control.
Conclusions
Drug induced liver injury must be considered as the first reason for an unexpected increase of ALT/AST activity in subjects with CF treated with CFTR modulators. However, the case of our patient shows that macroenzymes can emerge during CFTR modulator therapy, causing a biased increase of ALT/AST outputs in standard testing, mimicking important drug induced liver injury and leading to treatment discontinuation with loss of beneficial effect of CFTR modulator therapy. For that reason, macroenzymes should be considered as a potential reason for an unexpected increase of ALT/AST during CFTR modulator treatment.






