Introduction
Diabetes during pregnancy was first described by Benewith in 1826. Priscilla White (1900–1989) from Boston pioneered the development of the principles of proper treatment of maternal carbohydrate metabolism disorders. The classification of pregnant diabetic patients proposed by her is still used in a modified form (Hare and White) till today. In Europe, hyperglycemia is diagnosed in 13.7% of pregnant women (there is no epidemiological data on incidence of hyperglycemia in pregnant women in Poland) [1]. The frequency of gestational diabetes mellitus (GDM) worldwide is the highest in Southeast Asia (26.6%) [1]. After the introduction of new criteria by the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) in 2010 the frequency of GDM diagnosis increased 2–3 times. Hyperglycemia first diagnosed during pregnancy should be diagnosed and classified according to 2013 World Health Organization criteria for diagnosis of gestational diabetes (Table I). Globally, in 2021, an estimated 21.1 million or approximately 16.7% of births were adversely affected by diabetes in pregnancy. Of these, 80.3% were classified as GDM, 10.6% as diabetes detected before pregnancy including type 1 or type 2 diabetes mellitus and the remaining 9.1% were diabetes first detected during pregnancy (including type 1 and type 2 diabetes) [2]. Due to advances made in recent years in the field of neonatology, obstetrics and diabetology, perinatal mortality and the incidence of serious complications in IDMs have significantly decreased. Early diagnosis of gestational diabetes in accordance with the annually published recommendations of the Polish Diabetes Association and improvement of care for a woman suffering from diabetes in the pre-conceptional period can be considered a success [3]. In addition, thanks to The Great Orchestra of Christmas Charity, the provision of treatment options with personal insulin pumps was started in 2005, and in 2013 there was created a register of pregnant women with type 1 diabetes mellitus in pumps in Poland [1, 4]. From 2004 to the end of September 2022, 5,453 pregnant women benefited from this support [5].
Table I
Criteria for diagnosis Gestational Diabetes Mellitus (GDM) and Diabetes in Pregnancy (DiP) – based on Polskie Towarzystwo Diabetologiczne: Zalecenia kliniczne dotyczące postępowania u osób z cukrzycą 2023. Stanowisko Polskiego Towarzystwa Diabetologicznego. Curr Top Diabetes, 2023 [9]
Both pregestational and gestational diabetes are risk factors for severe neonatal morbidity and increased mortality [6, 7]. Abnormal blood glucose levels during pregnancy, whether due to diabetes already present before pregnancy or diagnosed during pregnancy, leads to increased incidence of neonatal complications [8]. Notwithstanding, presence of diabetes mellitus (DM) before pregnancy carries a greater risk of complications in the newborn. This may be due to the presence of vascular changes in type 1 and 2 diabetes and its longer duration than gestational diabetes. In the case of pregestational diabetes (type 1 or 2), proper glycemic control (HbA1c < 6.5%) in the pregestational period and in the first trimester of pregnancy significantly reduces the incidence of complications in the newborn [9–11].
The clinical presentation, recommended diagnostic and therapeutic procedures are described below.
Clinical presentation
Newborns of the mothers with pregestational diabetes and gestational diabetes look similar. They are usually large with increased body fat and enlarged internal organs [10]. Increased fetal insulin secretion leads to hypertrophy and hyper- plasia of the β cells of the pancreas, and to the growth of the newborn’s insulin-dependent organs like the liver, kidneys, skeletal muscles and the heart (except for the brain, whose cells take glucose from the blood without insulin) leading to organomegaly [12].
If maternal diabetes during pregnancy is well controlled, newborns may be born with a body weight between the 10th and 90th percentiles (appropriate for gestational age – AGA). If, on the other hand, mothers have additionally suffered from vascular complications of diabetes, their weight may be below the 10th percentile (small for gestational age – SGA). This is probably due to abnormalities in the placental vessels that may occur in diabetic mothers [6, 8]. Newborns who are macro- somic (large for gestational age – LGA; body weight above the 90th percentile), with fetopatic features on physical exam (broad shoulders with chest circumference larger than head circumference and organomegaly among other things) have a higher risk of perinatal injury because of shoulder dystocia and difficulties in delivering the fetus [6]. They are more prone to suffer from brachial plexus injury, or clavicular fracture. Asphyxia is also more common because their large size reduces the ability to tolerate stressful conditions, especially in the presence of concomitant cardiomyopathy.
Hypoglycemia develops in 5–27% of cases of IDMs [13]. Cord clumping after birth suddenly interrupts the glucose delivery to the newborn, which, with elevated fetal insulin levels, leads to hypoglycemia in the first hours after birth. The blood glucose level in the newborn declines to the lowest levels between first and third hour of life. Hypoglycemia may continue to be a problem for up to 72 hours and, in rare cases, even up to 7 days. Newborns with hypoglycemia usually present with tremors and appear anxious and hyperalert, but may also demonstrate hypotonia, lethargy and weakened sucking reflex. Those symptoms may be evident immediately after birth but may also occur later in the perinatal period. It should be emphasized that the same symptoms may result from hypocalcemia, hypomagnesemia or asphyxia. Hypocalcemia and/or hypomagnesemia secondary to transient parathyroid insufficiency usually appear in the first 24–72 hours [10, 12]. However, because in most cases, neonatal hypoglycemia is asymptomatic close glycemic control in the first hours and days of life is extremely important. It is also important to remember that elevated blood glucose levels in the umbilical cord and increased maternal fasting blood glucose levels increase the risk of hypoglycemia. Also perinatal asphyxia with increased irritability increases the risk of hypoglycemia.
When compared with newborns of non-diabetic mothers of comparable gestational age IDMs have a higher risk of developing respiratory distress syndrome (RDS). The greater risk is probably due to the antagonistic effect that the insulin has on cortisol (cortisol stimulates the synthesis of surfactant in the fetus, resulting in faster lung maturation) [10, 14]. Some reports, like secondary analysis of the Antenatal Late Preterm Delivery Steroids study (ALPS) showed that GDM was neither associated with an increased risk of the composite neonatal respiratory morbidity, nor with prolonged Neonatal Intensive Care Unit (NICU) admission or severe neonatal respiratory complications [15]. IDM often presents with tachypnea in the first few days of life. Apart from RDS other conditions, hypoglycemia, hypothermia, polycythemia, heart failure, transient tachypnea of the newborn and cerebral edema due to perinatal trauma or asphyxia should be considered in the differential diagnosis [15].
Fetal hyperinsulinism can induce polycythemia. IADPSG defines polycythemia in a term infant as hematocrit in a peripheral venous sample > 65% or hemoglobin > 22 g/dl [14]. This may result in a shift of iron from serum and storage pools to support increased erythropoiesis [16]. Although placental transferrin receptor protein expression is increased in diabetic pregnancies, the affinity of the receptor is reduced, resulting in no apparent increase in iron transport in spite of increased fetal iron demand and fetal tissue iron deficiency. Postnatally acquired iron deficiency causes long-term nonhematologic (heart, brain) organ dysfunction. IDMs are prone to abnormal cardiac and neurologic function in the newborn period and to long-term abnormal cognitive development [6, 8].
In 30% of cases, IDMs develop cardiomegaly, and in 5–10% of cases it progresses to heart failure [10]. Oxidative stress is suggested to play a role in myocardial complications developing in the first days of life [17]. Cardiomegaly is believed to be the result of glycogen being stored in the heart tissue under secondary to the effect of the increased glucose and insulin levels. Frequently occurring interventricular septal hypertrophy may manifest as transient idiopathic functional subaortic stenosis. In these cases, beta blockers release a narrowing that eventually subsides spontaneously. Inotropic drugs worsen the stenosis and are contraindicated [10].
Another rare serious complication of maternal diabetes is a renal vein thrombosis. It may be associated with polycythemia and iron deficiency conditions that are more common in IDMs. Renal vein thrombosis should be suspected in newborns with palpable tumors in the abdominal cavity, elevated blood pressure, hematuria and thrombocytopenia [10].
Incidents of hyperbilirubinemia are more common as a consequence of polycythemia and liver immaturity.
Some congenital malformations occur more commonly in IDMs and are associated with diabetic embryopathy. Birth defects are 2–3 times more common in newborns with pregestational diabetes than in the general population. Glucose can undergo autoxidation and generate reactive hydroxyl radicals (•OH) [18]. Hyperglycemia can cause increased generation of Reactive Oxygen Species (ROS). Overproduction of ROS induces protein oxidation, lipid peroxidation and different types of DNA damage [19].
The most common congenital anomalies in IDMs include central nervous system anomalies, mainly neural tube defects (cerebrospinal hernia, meningeal hernia, anencephaly) and cardiovascular defects (transposition of great vessels, ventricular septal defect, atrial septal defect, left heart hypoplasia, aortic stenosis, aortic coarctation) [20].
Other, less common birth defects include: caudal regression syndrome, small intestine atresia, renal agenesis, hydronephrosis, and polycystic kidneys. Small Left Colon Syndrome is a rare abnormality that develops during the second and third trimester of pregnancy. It is caused by changes in maternal glucose levels, hence in fetal levels which leads to impaired motility and intestinal development [21]. Prenatal ultrasound and a thorough physical examination identify these abnormalities [22].
Diagnosis
The complications of IDMs generally refer to newborns born to mothers with pre-gestational diabetes. Newborns born to mothers with DiP (diabetes in pregnancy – Table I) should be diagnosed as newborns born to mothers with pregestational diabetes (PGDM).
Blood glucose should be monitored in all IDMs. Monitoring of hematocrit, electrolytes and ultrasound of the heart should be performed in newborns of mothers with pre-gestational diabetes and diabetes diagnosed in the first trimester of pregnancy (DiP), as well as in newborns of mothers with gestational diabetes showing significant macrosomia and features of diabetic fetopathy.
The algorithm for the diagnosis of IDM according to the Recommendations of the Polish Neonatal Society [23] is presented below:
Blood glucose level assessment – 1–2 hours after birth, and then every 4 hours until the end of the 24 hours of life. Monitoring of blood glucose can be completed earlier if the child is willing to suckle, shows no abnormalities on the clinical examination, and the results of two consecutive blood glucose measurements are normal.
Determination of the hematocrit after completing the first day of life.
Assessment of the concentration of calcium and magnesium in the case of tremors or other symptoms of electrolyte disturbances, alternatively, as a standard, after completing the first day of life.
Determination of bilirubin concentration when jaundice is visible. Treatment with phototherapy – according to general guidelines.
Ultrasound screening imaging examinations for congenital malformations of internal organs – heart, urinary system, central nervous system are not recommended as standard. Most significant birth defects are detected prenatally by ultrasound. In the absence of prenatal diagnosis, screening imaging should be considered in newborns of mothers PGDM and DiP.
It is recommended to perform an ultrasound of the heart in order to detect hypertrophic cardiomyopathy and possible small congenital heart defects – before discharge from the hospital (around the third day of life).
Newborns diagnosed with hypertrophic cardiomyopathy should be referred to a pediatric cardiologist. Monitoring should continue until the muscle hypertrophy resolves.
Treatment
Because the plasma glucose concentration cut-off values that cause hypoglycemic brain damage and the effect of asymptomatic hypoglycemia on neurodevelopment are unknown, the use of guidelines is currently the most appropriate method to maintain safe blood glucose levels [24]. IDM should be fed within the first hour after birth. The first glucose screening should be performed within 30 minutes after the first feeding. Target glucose values are ≥ 40 mg/dl before feeding during the first 48 hours of life [25]. The physician must evaluate the clinical condition and the metabolic disturbances and consider them when treating hypoglycemia. Treatment is indicated when glucose levels are below 45 mg/dl. Feeding is the initial treatment for asymptomatic hypoglycemia. Feeding with either breast milk or modified milk. The Canadian Paediatric Society recommends the applying intrabuccal 40% dextrose gel [26], although early feeding is equally effective. Recurrent hypoglycemia may be treated with repeated feedings or intravenous glucose infusion. Persistent hypoglycemic infants (glucose levels < 25 mg/dl in the first 4 hours postpartum and < 35 mg/dl between 4 and 24 hours postpartum and no expected increase in glucose after oral administration) need to be treated with an intravenous glucose infusion especially when symptoms of hypoglycemia occur [27]. A bolus of 200 mg/kg glucose (2 ml/kg of 10% glucose) should be administered followed by continuous glucose infusion to avoid recurrence of hypoglycemia [28]. If there are doubts as to whether the newborn can tolerate oral feeding, despite the fact that it sucks readily, an intravenous infusion with 10% glucose ought to be administered at a rate of 4–8 mg/kg/min [29]. Neurological symptoms of hypoglycemia have to always be treated with intravenous glucose infusion. Boluses of hypertonic glucose (20%) should be avoided as they may cause further hyperinsulinemia and potentially rebound hypoglycemia. In case of hypomagnesemia [magnesium concentration < 1.5 mg/dl (< 0.6 mmol/l)], 20% magnesium sulphate should be administered intravenously in a dose of 0.1–0.3 ml/kg, while in case of hypocalcemia [total calcium < 7 mg/dl (< 1.8 mmol/l)] – 10% calcium gluconate 1–2 ml/kg, followed by an oral maintenance dose of 50–60 mg/kg/day [23].
Neonatal hypoglycemia values requiring intervention according to AAP, PES, BAPM and the algorithm for the treatment of IDM according to the Clinical Report – Postnatal Glucose Homeostasis in Late-Preterm and Term Infants Pediatrics (2011) are presented in Table II and Figure 1 [29, 30].
Figure 1
Screening for and Management of Postnatal Glucose Homeostasis in IDM – based on Adamkin DH. Clinical Report – Postnatal Glucose Homeostasis in Late-Preterm and Term Infants Pediatrics, 2011 [29]
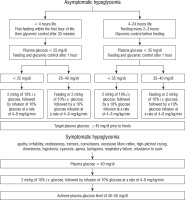
Table II
Neonatal hypoglycemia tresholds requiring intervention according to AAP, PES, BAPM – based on Cassertano A, Rossi A, Fecarotta S, et al. An Overview of Hypoglycemia in Children Including a Comprehensive Practical Diagnostic Flowchart for Clinical Use. Front Endocrinol (Lausanne, 2021) [30]

 POLSKI
POLSKI






