Summary
Infarct size (IS) governs left-ventricular end-systolic and end-diastolic volume changes after acute myocardial infarction, and it is a strong predictor of adverse long-term prognosis. Therefore, it would be clinically desirable to inhibit the effect of infarct size on left-ventricular remodelling. An important role might be played by cell-based therapies that have the potential to enhance cardiac repair. However, very few studies have used labelled cells, and cohorts have been very small; thus, the impact of progenitor cell myocardial uptake on the relationship between infarct size and left ventricular remodelling has not been explored. In the largest study to date with labelled stem cells in ST-segment elevation myocardial infarction, we found that cell uptake (occurring in proportion to IS) attenuated the effect of infarct size on ΔESV and ΔEDV at 2 years. Furthermore, we found a significant improvement in left ventricle systolic function expressed by circumferential strain on cardiac magnetic resonance imaging, which was particularly relevant in the medium tercile of large-size infarcts. To boost the effect that we identified, further cell-based strategies to stimulate cardiac repair in acute myocardial infarction should involve cell types and delivery techniques to maximize myocardial uptake.
Introduction
Despite progress in medical and device therapies, post-myocardial infarction (MI) heart failure continues to be associated with reduced quality of life and poor prognosis [1]. The number of patients with ischaemic heart failure is prognosed to significantly grow in the next decades [2, 3], prompting development of novel therapeutic strategies [3]. Cell-based approaches receive increasing attention [4, 5] and attract significant research efforts [6–14].
There is ample evidence that infarct size (IS) is a fundamental determinant of left-ventricular (LV) adverse remodelling (i.e. adverse change in LV geometry after MI) [15–18]. LV adverse remodelling is typically expressed as an increase in end-systolic and end-diastolic volumes over time [19]. Another important index of the degree of ischaemic myocardial damage and reduced function over time is circumferential myocardial fibre shortening/deformation (strain) [20, 21]. Recent evidence shows that circumferential strain (CS) may add an important diagnostic and prognostic value over that of conventional (i.e. LV volume-dependent) parameters of LV remodelling [22, 23].
Aim
Our prior work demonstrated that IS determines the magnitude of myocardial uptake of transcoronary-delivered progenitor cells [24] consistent with biologically relevant mechanism(s) governing the relationship between IS and cell uptake [24]. Presently, we have evaluated the relationship between IS, progenitor cell uptake, and the magnitude of LV remodelling in patients receiving transcoronary administration of progenitor cells shortly after MI.
Material and methods
The study involved consecutive patients with ST-segment elevation myocardial infarction (STEMI) (anterior location, left anterior descending artery, LAD, as the infarct-related artery), treated with primary percutaneous coronary intervention (pPCI) and with persistent left ventricle ejection fraction (LVEF) ≤ 45% by echocardiography (Simpson method) and cardiac necrosis biomarker level consistent with major myocardial loss (peak troponin I (TnI) level ≥ 50 ng/ml and/or peak creatine kinase-myoglobin binding (CK-MB) ≥ 150 U/l with no contraindication to cardiac magnetic resonance imaging (cMRI). Patients were recruited to meet the volume goal of at least 30 subjects [24]. Bone marrow (BM) aspiration was performed 7 to 12 days (median 10 days) after pPCI. On the morning of the day of cell administration, BM (80–120 ml) was harvested from iliac crest. Mononuclear cells were separated with Ficoll [10, 25]. Total amount of 0.7–9.9 (median 4.3 × 106) autologous CD34+ cells (50% 99mTc-extametazime-labeled), including 0.3–6.8 (median 2.1 × 106) CD34+CXCR4+ cells, were administered intracoronarily (non-occlusive technique) via the infarct-related artery (LAD). Myocardial perfusion was evaluated by single-photon emission computerized tomography (SPECT) 36 to 48 h before cell delivery. Myocardial uptake of the labelled CD34+CXCR4+ cells was assessed 1 h after transcoronary cell implantation by whole-body planar γ-camera scan. Cell uptake was quantified by the number of counts in the cardiac region of interest in relation to total counts on whole-body images [24, 26].
Gadolinium-enhanced cMRI (Siemens Magnetom Sonata 1.5 T) with image acquisition ≤ 24 h before cell transfer was used as the reference technique for the detection and assessment of MI size and parameters of remodelling (LV end-diastolic volume (EDV), LV end-systolic volume (ESV)). CMR parameters were analysed with dedicated software (QMass MR 7.5; Medis, Leiden, the Netherlands) by agreement of 2 observers with at least 5 years of experience in core lab cMRI analysis. Late gadolinium-enhanced (LGE) images were obtained 10 to 15 min after a peripheral bolus injection of 0.2 mmol/kg Gd-DTPA [24]. Gadolinium late-enhanced total infarct mass/size (IS, cMRI) included both the core zone (defined as signal intensity (SI) ≥ 50% of the maximal myocardial SI) and the infarct border zone (defined as the myocardium with SI greater than the peak SI in remote normal myocardium but < 50% of maximal SI of the high SI myocardium outward to the LGE zone). As a result, total IS was defined as total myocardium with signal intensity greater than SI peak in remote normal myocardium [24].
Furthermore, mid CS (mCS) analysis was conducted as previously described [21, 27]. In brief, using Diogenes CMR-FT software (TomTec Imaging Systems, Munich, Germany), peak systolic circumferential strain at infarct zone (LV mid zone) was measured in LV short axis views at mid for baseline and follow-up. Segmental strain analysis was not performed for apical segments due to low reproducibility, and not for basal segments due to the absence of infarct tissue. CS was measured in mid anteroseptal, anterior, and anterolateral segments if there were LGE present (defined as in core or border infarct zone above). The MRI measurements were taken by a consensus of 2 independent, experienced cMRI analysts blinded to the SPECT and clinical data. cMRI imaging, with measurements of EDV, ESV, and mCS, was repeated at 24 months. Change in EDV (ΔEDV) and ESV (ΔESV) was calculated along with a comparison of mCS at baseline and at 24 months. To evaluate the relationship between IS, progenitor cell uptake, and the magnitude of LV remodelling, IS was categorized into terciles (g). The values of median cell uptake along with ΔESV, ΔEDV, and the mCS data were calculated in each IS tercile. The study was approved by the Local Ethics Committee, and all participants provided informed written consent.
Statistical analysis
Variables were presented as numbers and percentages, mean ± standard deviation (SD), or median and interquartile range, as appropriate. Data distribution was assessed via the Shapiro-Wilk test. Categorical variables were compared using Pearson’s χ2 test or Fisher’s exact test. Comparison of variables between terciles was performed using ANOVA. Differences between 2 groups (baseline versus 24-months follow-up) were compared using the Wilcoxon test. Spearman’s correlation coefficients were used to determine trends. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed with JMP 15.2 (SAS Institute Inc., Cary, NC, USA, 2020).
Results
Clinical and laboratory characteristics of the study group are shown in Table I. Median peak TnI level was 137 ng/ml (58.3–356 ng/ml), and median peak CK-MB was 584 IU/l (181–962 IU/l). Median values of LVEF were 37% (23–44%) by echocardiography, 34% (17–48%) by gated SPECT (G-SPECT), and 38% (21–48%) by cMRI. One hour after transcoronary cell delivery, myocardial activity uptake was 1.7% to 9.9% (median 5.2%). Total infarct mass (cMRI) was 57 [11–112] g.
Table I
Clinical characteristics of studied patients
| Variable | Value median, (range) or proportion |
|---|---|
| Number of patients, n | 31 |
| Age [years] | 58 (36–69) |
| Time from the onset of symptoms to pPCI [h] | 6 (3–13) |
| Infarct-related artery = proximal LAD, n (%) | 31 (100) |
| Peak CK-MB [IU/l] | 584 (181–962) |
| Peak troponin I [ng/ml] | 137 (58.3–356) |
| Hypertension, n (%) | 14 (45.2) |
| Diabetes mellitus, n (%) | 7 (22.6) |
| Hyperlipidaemia, n (%) | 26 (83.9) |
| Smoking (history or current), n (%) | 16 (51.6) |
| BMI > 30 kg/m2, n (%) | 2 (6.4) |
| eGFR < 90 ml/min/m2 | 8 (25.8) |
| pPCI to cell transfer [days] | 10 (7–12) |
| LVEF by echocardiography* (%) | 37 (23–44) |
| LVEF by G-SPECT* (%) | 34 (17–48) |
| LVEF by cMRI* (%) | 38 (21–48) |
| Myocardial uptake of cell label# (%) | 5.2 (1.7–9.9) |
pPCI – primary percutaneous coronary intervention, LAD – left anterior descending artery, CK-MB – creatine kinase-myocardial band, BMI – body mass index, eGFR – estimated glomerular filtration rate, LVEF – left ventricular ejection fraction, G-SPECT – gated single-photon emission computerized tomography, cMRI – cardiac magnetic resonance imaging.
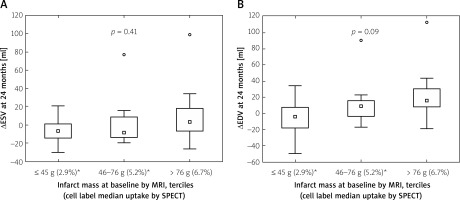
Cell label myocardial uptake (whole-body γ-scans) was proportional to IS (r = 0.62), with a median 2.9% uptake in IS 1st tercile (≤ 45 g), 5.2% in 2nd (46–76 g), and 6.7% in 3rd (> 76 g) (p = 0.0006). At 24 months EDV increased by a median 7.7 ml whereas ESV decreased by a median 6.0 ml. ∆ESV and ∆EDV in each IS tercile are depicted in Figure 1. There was no statistically significant relationship between IS and ∆ESV (p = 0.41) or between IS and ∆EDV (p = 0.09), suggesting that the cell uptake in proportion to IS may have abolished the effect of IS on ΔESV and attenuated the effect of IS on ΔEDV.
Figure 1
Relationship between infarct mass at baseline, cell uptake, and long-term LV adverse remodelling. Infarct size (IS) is categorized by terciles of infarct mass; ≤ 45 g (1st tercile), 46–76 g (2nd tercile), and > 76 g (3rd tercile); median cell label myocardial uptake by respective IS terciles is provided in parenthesis in the bottom line. Left panel shows ∆ESV at 24 months by the IS and cell uptake whereas ∆EDV at 24 months by the IS and cell uptake is demonstrated in the right panel. Note lack of a statistically significant increase in ∆ESV and ∆EDV at 24 months in relation to IS. As IS is a fundamental determinant of ∆ESV and ∆EDV [15–18, 28], this finding suggests that progenitor cells, attracted to the infarct zone in relation to the IS, may attenuate the adverse effect of IS on LV remodeling
ESV – end-systolic volume, EDV – end-diastolic volume, ∆ – change, MRI – cardiac magnetic resonance imaging, SPECT – single photon emission computed tomography.
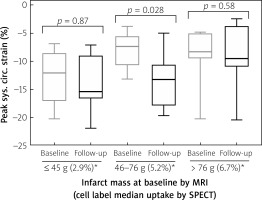
At baseline, median mCS was –12.1% in IS 1st tercile; –7.4% in 2nd tercile, and –8.3% in 3rd tercile (p = 0.04, more severe contractility impairment in the 2nd and 3rd IS tercile than in the 1st IS tercile, Figure 2 A). cMRI follow-up at 24 months showed mCS medians of, respectively, –15.45%;–13.3% and –9.55% (p = 0.14, indicating attenuation of the effect of IS, Figure 2 B). At 24 months, mCS improved in IS 2nd tercile (p = 0.028) while it revealed no significant change in smaller (p = 0.87) or larger infarcts (p = 0.58) (Figure 3).
Figure 2
Relationship between infarct mass at baseline, cell uptake, and peak systolic circumferential strain at baseline (A) and 24-month follow-up (B). Infarct size (IS) is categorized by terciles of infarct mass; ≤ 45 g (1st tercile), 46–76 g (2nd tercile), and > 76 g (3rd tercile). Median cell label myocardial uptake by respective IS terciles is provided in parentheses in the bottom line. Figure 2 A shows peak systolic circumferential strain at baseline (< 24 h before cell administration) in mid segments (infarct zone). Note a statistically important difference in peak systolic circumferential strain between the terciles (p = 0.04); consistent with a relationship between infarct size and circumferential strain (i.e. the larger the infarct, the worse the myocardial strain). Figure 2 B shows peak systolic circumferential strain at 24 months (< 24 h before cell administration), in mid segments, inside the infarct zone. Note the lack of a statistically significant difference between the peak systolic circumferential strain terciles (p = 0.14), suggesting attenuation of the effect of IS on contractility deterioration as expressed by the strain
MRI – cardiac magnetic resonance imaging, SPECT – single photon emission computed tomography.
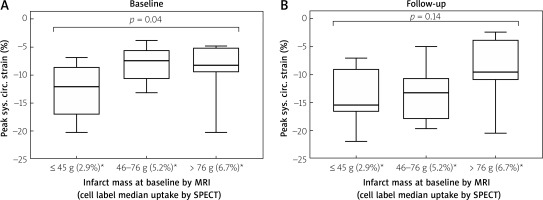
Figure 3
Relationship between the baseline and 24-month follow-up peak systolic circumferential strains in mid segments (infarct zone). Infarct size (IS) is categorized by terciles of infarct mass; ≤ 45 g (1st tercile), 46–76 g (2nd tercile), and > 76 g (3rd tercile). Note a significant improvement of strain in 2nd tercile of IS (p = 0.028), which may suggest a beneficial impact of progenitor cell administration, which is particularly relevant in this subgroup
MRI – cardiac magnetic resonance imaging, SPECT – single photon emission computed tomography.
Discussion
The principal findings from this largest study to date evaluating the relationship between IS, progenitor cell uptake, and the magnitude of LV remodelling in patients receiving transcoronary administration of progenitor cells shortly after MI, suggest the following: (1) stem cell uptake, proportional to IS, may attenuate the established effect of IS on ∆ESV [16, 28] and may inhibit the established effect of IS on ∆EDV [15, 17, 18, 28] (Figure 1), and (2) cell therapy with autologous CD34+/CD34+ CXCR4+ cells may improve LV systolic function (expressed by mCS strain on cMRI), in particular the medium tercile of large infarcts (Figure 3).
IS, measured preferably by cMRI, is a well-established determinant of adverse LV remodelling after myocardial infarction [15–18, 29]. Marked LV remodelling, despite its role in maintaining overall stroke volume, leads to clinical heart failure presentation in a large proportion of patients. IS is also prognostic of adverse clinical outcomes, an effect significant also in multivariate analysis [16, 29], and it is a predictor of follow-up LVEF [16]. On the other hand, it is important to realize that IS, as derived from cMRI late gadolinium enhancement (LGE), does not necessarily equate with irreversible injury because it recedes over time [30]. Some parameters other than the baseline infarct size by cMRI-LGE may add an incremental prognostic value [23]. Recently, increasing evidence has emerged on the role of myocardial deformation parameters such as strain, which is reflective of myocardial fibre shortening [23]. Strain can be evaluated on echocardiography or, more reproducibly, on cMRI [20]. Circumferential strain (CS) measured by feature tracking technique is an important modern parameter of LV systolic function [20]. CS is at least equal (and it may be superior) to traditional cMRI measures in the assessment of cardiac regenerative therapy [22] because it may help to evaluate segmental improvement [31] and late myocardial remodelling [32]. Some [33] (though not all [34]) authors argue that CS may provide an additional long-term prognostic value in STEMI patients and improve risk reclassification beyond traditional cMRI indexes. One advantage of this method is the absence of the need for administration of contrast agent [35]. Our findings (Figure 2) suggest that stem cell therapy may improve LV systolic function (as measured by mCS) particularly in medium-size infarctions. To-date, data on cMRI strain analysis in cell therapy trials are limited [21]; thus, our study adds significantly to the existing body of evidence.
The therapeutic effect of stem cells is based on myocardial uptake as a primary requirement for any therapeutic action [6, 11, 13, 14, 36, 37]. Dill et al. [38] indicated that autologous bone marrow cell administration shortly after STEMI improved LVEF, reduced EDV, and abrogated ESV increase after 12 months, particularly in patients with lower LVEF values (larger infarcts). In a recent meta-analysis, transcoronary transplantation of bone marrow progenitor cells was safe and induced a significant increase in LVEF with a trend towards ESV reduction and fewer cardiac adverse events [39]. Nevertheless, myocardial uptake of bone marrow cells appears to be, overall, rather low (median of 5.2% in our study, ranging from ≈2.1% to 9.2% in other studies) [40, 41]. It is therefore important, as a future research direction, to evaluate therapeutic potential of other cell types, particularly those that exhibit retention in the ischaemic injury zone [42, 43] greater than bone marrow cells [4].
There is ample evidence on the relationship between baseline infarct size and follow-up ejection fraction [28, 44] and adverse remodelling [18, 28], especially in patients with larger infarcts [17, 29]. Infarct size, in the absence of cell therapy, is also a stronger predictor of all-cause mortality than LVEF and LV volumes [45]. Our present study indicates that with stem cell uptake in proportion to IS, IS may no longer be a determinant of the magnitude of LV adverse remodelling after infarction, consistent with a potential role of stem cells in enhancing myocardial repair in recent STEMI.
Despite being the largest to-date study with labelled progenitor cell transplantation shortly after STEMI in humans, our overall patient volume may be considered moderate. Thus, larger studies are required to corroborate our present findings. Furthermore, while there is no doubt that a parallel sham/placebo comparative cohort would be desirable [8, 46], the sample size in our study of labelled cells did not justify a control sham/placebo group.
Conclusions
This largest human study with labelled CD34+ cell transplantation shortly after MI suggests that the cell uptake, occurring in relation to IS, may attenuate the effect of IS on LV adverse remodelling. To boost this effect, further strategies should involve cell types and delivery techniques maximizing myocardial uptake.








