Introduction
Tissue adhesives, also known as local hemostatics or sealants, are used in combination with conventional surgical methods to reduce blood loss, decrease the cost of surgery, and make the surgery safer to perform. They are often used in cardiac surgery where there is an extremely high risk of excessive blood loss. The scientific evidence in clinical practice is not sufficient to create standardized guidelines for their use; thus there is great variation in their use from center to center [1]. BioGlue (Cryolife Inc., Kennesaw, GA) is a widely used adhesive characterized by high fluidity and high adhesion strength to tissues. The described sealant is a two-component adhesive that consists of 45% bovine serum albumin and 10% glutaraldehyde. Especially the aforementioned glutaraldehyde can cause complications associated with the use of this agent, such as inflammatory reaction, nerve paralysis, and necrosis of surrounding tissues [2]. The Food Drug Administration (FDA) approved the described sealant in December 2001 for use in cardiac and vascular surgery. Since then, this product has been used successfully worldwide in many types of procedures [3, 4]. Some examples are presented in Table I [5–10]. BioGlue is mainly used in major cardiac and vascular surgery procedures, including acute aortic dissection, where it significantly increases short-term patient survival [11]. However, there are concerns about its long-term impact on patients due to the additional induction of inflammation [11]. Apart from BioGlue, there are other tissue adhesives in use, such as fibrin sealant. Some recent studies confirmed the effectiveness of its application in cardiac surgery, while other authors report the lack of effectiveness of fibrin sealant in reducing the length of hospitalization of patients after pulmonary resection [12, 13]. Comparing the effectiveness of BioGlue to other adhesives appears to be a promising area of research, but it is beyond the scope of this paper.
The overarching criterion and the object of interest of this paper is the safety associated with the use of BioGlue in cardiovascular surgery. Potential side effects in the form of inflammation, which in some cases can be a life-threatening condition, are preventable with proper use of the described sealant. However, it is important to be aware of potential inflammatory complications, which are the subject of this paper.
PubMed, PubMed Central and Google Scholar online databases were searched using terms related to: “BioGlue”, “hemostats”, “adhesive sealants in cardiac surgery”, “BioGlue complications” and “BioGlue inflammation response”. After reviewing 60 abstracts, articles describing the effects of the described product on representative groups of patients were selected whenever possible. The types of articles reviewed were original papers, meta-analyses, review papers and case reports. Additionally, chapters from English-language books were used to complete the overall review described.
Mechanism of inflammatory response
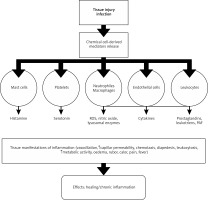
A recent textbook of basic pathology cites a definition of inflammation as the response of vascularized tissues to infection or damage, attracting cells and the organism’s defense molecules to the location of the harmful agents in order to eliminate them [14]. However, the exact definition of this phenomenon is a bit more complicated. As pointed out by Antonelli and Kushner, many conditions commonly classified as inflammatory do not fully meet the conditions proposed above. The authors cite gout and autoimmune diseases because their cause is often not infection or tissue damage [15]. This highlights the complexity of the topic of inflammation. Basic inflammatory mediators, or substances responsible for the initiation and regulation of the immune response, include histamine, prostaglandins, leukotrienes, cytokines (TNF, IL-1, IL-6), chemokines, reactive oxygen species (ROS), platelet activation factor (PAF), complement system components and kinins. Their activity leads to the development of a systemic response, the so-called acute phase. Its most important components are fever, C-reactive protein (CRP) increase, leukocytosis and others [14]. Systemic inflammation is also associated with a response from the endocrine and nervous systems. Changes in blood hormone levels mainly involve systems responsible for the balance between anabolism and catabolism and an increase in the activity of the renin-angiotensin-aldosterone system [16]. To assess the effect of the use of surgical adhesives in cardiovascular surgery on the development of the inflammatory response, the selection of appropriate inflammatory markers is crucial. The most commonly used biochemical markers include cortisol levels, IL-6, white blood cell (WBC) counts and CRP levels [17]. The pathogenesis of the inflammatory response is presented in Figure 1.
Clinical complications of sealant applications
In addition to its advantages, the application of BioGlue can also cause clinical complications. A study described by Luk et al. histopathologically demonstrated an inflammatory reaction after using BioGlue. The polymerized sealant can release glutaraldehyde, which has cytotoxic effects on adjacent cells [18]. This chemical compound appears to be one of the main inducers of complications after using BioGlue.
Vascular inflammation and necrosis
BioGlue induced inflammation of the aortic tissue and caused the occurrence of edema and necrosis at the application site [19]. Its use as part of the treatment of acute aortic dissection type A (AADA) can result in vascular necrosis [20, 21]. BioGlue is used for aortic root reconstruction. During surgery, it is not always possible to apply the glue perfectly to the target site and residues spread beyond the application site, causing cytotoxic effects on adjacent cells. In this study, rupture of a pulmonary artery was observed as a result of a chemical reaction involving the sealant. Small amounts of glue applied to the aortic root spread to the pulmonary arteries and caused a massive inflammatory reaction with histiocytes and granulocytes [22–24]. There have been conducted studies whose conclusions indicate that a small amount of BioGlue released from the wound can only cause low-to-medium-grade inflammation of aortic tissue [25, 26]. In addition, it can also cause high-grade inflammation and necrosis in the lungs and liver. BioGlue can have cytotoxic effects and induce a foreign body type immune response [19]. As can be seen, despite the manufacturer’s preparation of BioGlue for precise application, complications can occur due to adhesive leakage outside the operated area.
Bacterial mediastinitis
Erasmi et al. reported the development of severe inflammation observed 3 months after the procedure around the BioGlue application site in an aortic arch reconstruction. The glue acted as an additional focus of inflammation and thus caused the formation of increased amounts of mediastinal fibrosis [27]. Along with inflammation, patients experienced symptoms such as pain and discomfort. However, there is a risk that such a large inflammatory mass will form, resulting in a situation as serious as mediastinitis. The described sealant residues undergo proteolytic degradation. Unfortunately, there are also situations of incomplete degradation of the glue and thus it remains in the tissues beyond the application site, becoming a breeding ground for bacterial infection [28]. There was described a case of a 67-year-old patient with a tumor in the neck on the left side, 4 years after carotid endarterectomy with BioGlue application. Markers of inflammation were normal but surgical exploration revealed cavities within the operated vessel that were filled with pus and a black gel-like substance. Histological examination revealed that this was a response of histiocytes to the infected acidophilic material and the residual glue was colonized by Staphylococcus aureus [29].
Cardiac tamponade
It is not possible to completely remove the product after direct application to the myocardium without causing severe damage. Therefore, the glue in the myocardium can cause chronic inflammation, which is then untreatable. A case has been described of a patient who developed such a reaction leading to cardiac tamponade caused by a chronic granulomatous inflammatory response to the glue. Pericardial effusion with signs of tamponade developed 5 months after aortic valve reconstruction procedure and BioGlue application. The patient was treated by the removal of all foreign material and BioGlue [30].
Formation of tumors and cysts
A 66-year-old patient underwent coronary artery bypass grafting (CABG) in which BioGlue was used to strengthen the lower heart wall. 5 months after surgery, a tumor appeared in the lower sternal incision line. A computed tomography (CT) scan showed a fluid mass in the mediastinum compressing the heart. After draining, fragments of polymerized sealant were extracted from a cavity located next to the lower wall of the right ventricle. After removal of the glue fragments, the cavity spontaneously healed [31]. There is also described a case of another 66-year-old patient who developed a giant mediastinal cyst 7 months after cardiac surgery, during which BioGlue was used to strengthen the sutures. The patient consulted a physician 7 months after surgery because of cyst formation at the subcostal incision site. A suspicion of mediastinitis was raised. Thoracoscopic techniques were able to drain the cyst and clinicians found clear fluid and a dark, hard, irregular mass of artificial material (2 cm in diameter) in the sternal cavity. A foreign body type inflammatory reaction to the described sealant could potentially develop in other mediastinal areas such as large vessels and represent a life-threatening condition [28].
Wound healing impairment
BioGlue is occasionally used during transcatheter aortic valve implantation (TAVI) mostly during transapical access. There have been described cases of 3 patients who experienced late wound healing problems between the apex and the anterior thoracic wall after TAVI that were caused by the described sealant. During revision, an inflammatory reaction to the foreign body surrounding healthy vascularized tissue was noted. All intraoperative cultures were sterile, although purulent material was noted during wound debridement [32].
Pseudoaneurysm
BioGlue may contribute to the formation of a pseudoaneurysm by the mechanism of a prolonged inflammatory response. Mechanical stress, vasculitis, infection, damaged anastomoses and the material used are important factors in the pathogenesis of a pseudoaneurysm [33]. The authors suggest that BioGlue alone should not be applied to strengthen the sutures during aortic dissection repair, but suggest its use together with Teflon strips, as then pseudoaneurysm formation is very rare [34, 35]. The authors suggest that the use of the sealant should be limited to aortic dissection surgery, as any other tissue is sensitive to the harmful effects of glutaraldehyde [19, 36].
Conclusions
The use of the described tissue glue is common in cardiac surgery and is associated with a significant improvement in hemostasis. However, the amounts of BioGlue should be dosed very carefully and adequate time should be allowed to bind to the tissues, significantly reducing adverse effects of inflammation.