Introduction
The adaptive response to viral infections, which includes both B-cell-based humoral immunity and T-cell-based cellular immunity, can be influenced by various factors, including age, sex, disease severity, comorbidities, and medicines. For example, it is more frequently deteriorated in elderly individuals due to immunosenescence [1], males due to different effects of sex hormones on the immune system [2], and immunosuppressed patients [3], while asymptomatic or mildly infected subjects may mount different humoral and cellular adaptive responses than those who are severely or critically ill [4].
There is also some recent evidence that influenza vaccination may increase the strength of the adaptive response to other viral infections. As demonstrated, receiving concomitant pneumococcal and influenza or only influenza vaccine resulted in a higher concentration of antibodies against the SARS-CoV-2 receptor binding domain (RBD) and increased micro-neutralization titers following administration of COVID-19 mRNA vaccine [5, 6]. Moreover, COVID-19 convalescent patients who previously received seasonal influenza vaccination were characterized by higher seroprevalence and concentrations of immunoglobulin (Ig) G antibodies against RBD and nucleocapsid protein [7]. The exact mechanisms behind such an effect remain to be elucidated. However, it is hypothesized that it may arise from vaccination-driven interleukin (IL)-4 production by T-helper 2 cells that leads to improved clonal expansion of B cells as well as elevated IL-5 and IL-6 production, which play a role in the later phase of B-cell activation by supporting their differentiation and antibody production [7, 8]. Moreover, it is suggested that influenza vaccination can induce innate immune training in myeloid cells by altering cytokine production through epigenetic reprogramming of transcriptional pathways, ultimately leading to better support of adaptive humoral responses [9–11]. Various observations indicate that such trained immunity may exist in the context of the influenza vaccine. Individuals who received seasonal vaccination were characterized by lower exposure to seasonal alphacoronaviruses and decreased risk of SARS-CoV-2 infection, hospitalization, need for mechanical ventilation, and death due to COVID-19 [12–15].
If one considers that influenza vaccination improves heterologous adaptive humoral responses to SARS-CoV-2, this effect is likely to occur not only in relation to the production of IgG antibodies, which are generated later, but also immunoglobulins produced in the earlier phases. It is evidenced that contrary to various other viral infections, IgA antibodies, instead of IgM, dominate the early phase of the humoral response to SARS-CoV-2 due to their preferential expression by circulating plasmablasts [16, 17].
Therefore, the present study compared SARS-CoV-2-specific IgA responses in a large group of COVID-19 convalescents who were not vaccinated against COVID-19 but had a primary SARS-CoV-2 infection and differed in the status of the seasonal influenza vaccine. This is the first study to explore the potential association between influenza vaccination and humoral responses to SARS-CoV-2 infection in relation to this antibody class, which, contrary to IgG and IgM studies, was often not included in the serosurveillance investigations conducted in the early phases of the COVID-19 pandemic.
Material and methods
Collection of serum samples
All serum samples for this study were purchased in 2020 from Regional Blood Donation and Blood Treatment Centers located across Poland in nine voivodeships (Greater Poland, Kuyavia-Pomerania, Lesser Poland, Lower Silesia, Łódź, Masovian, Podlaskie, Silesia, Western Pomerania), according to accepted safeguard standards and legal requirements. All samples were collected between September and December 2020 from individuals with a history of SARS-CoV-2 infection confirmed with RT-PCR and one month after the resolution of COVID-19 symptoms/end of the isolation period. This period was dominated by infections with Nextstrain clades 20A, 20B, and 20C [18], which did not show significant differences in clinical outcomes [19, 20]. Until the end of 2020, 1.2 million SARS-CoV-2 infections were confirmed in Poland; the majority of these (approx. 95%) were detected in September-December 2020 [21]. Therefore, it can be assumed that most, if not all, studied individuals experienced primary SARS-CoV-2 infection, particularly if one considers that this period was characterized by various social distancing and isolation measures and a low level of reinfections [22].
In total, 1318 serum samples were purchased, of which 659 were collected from individuals vaccinated against influenza during the 2019/2020 epidemic season and 659 from unvaccinated persons. All influenza-vaccinated individuals received the vaccine in the recommended period between September and December 2019, approximately one year prior to confirmed infection with SARS-CoV-2. An inactivated quadrivalent influenza vaccine used in the 2019/2020 season in the Northern Hemisphere consisted of the following antigens: (i) an A/Brisbane/02/2018 (H1N1)pdm09-like virus, (ii) an A/Kansas/14/2017 (H3N2)-like virus, (iii) a B/Co-lorado/06/2017-like virus (B/Victoria/2/87 lineage), and (iv) a B/Phuket/3073/2013-like virus [23]. The data regarding age, sex, and severity of SARS-CoV-2 infection in studied individuals were gathered by the Regional Blood Donation and Blood Center. Patients who reported not having any symptoms of infection were classified as asymptomatic, those who were symptomatic but did not require hospitalization were considered to have mild disease, and cases of patients whose symptoms required hospitalization were defined as severe. All studied individuals experienced primary SARS-CoV-2 infection. None of the studied individuals were vaccinated against COVID-19 since the samples were collected in the last quarter of 2020 because the vaccines had not yet been authorized.
The study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethical Committee of the Institute of Public Health – National Research Institute (protocol code 4/2020, 6 August 2020) and the Bioethics Committee at the Poznan University of Medical Sciences (protocol code 429/22, 11 May 2022).
Determination of anti-SARS-CoV-2 IgA antibodies
The prevalence and concentration of three anti-SARS-CoV-2 IgA antibodies were determined in the present study: IgA against nucleocapsid protein (anti-NP), receptor-binding domain of the spike protein (anti-RBD), and subunit S2 of the spike protein (anti-S2). All antibodies were determined using the CE-IVD certified Microblot-Array COVID-19 IgA Assay (TestLine Clinical Diagnostics). In this assay, recombinant and purified native antigens are immobilized on specific spots of nitrocellulose membrane fixed at the bottom of the microplate well [24]. The concentrations for all three IgA antibodies were given as U/ml and interpreted as positive if > 210 U/ml.
Statistical analyses
The statistical analyses were performed using Statistica v.13.1 (StatSoft) and MedCalc 15.8 (MedCalc Software). The differences in the seroprevalence of antibodies against SARS-CoV-2 between particular sub-groups were analyzed with Pearson’s chi-square test. Differences in antibody concentrations were assessed with the non-parametric Mann-Whitney U test (comparison of two groups) or Kruskal-Wallis ANOVA (comparison of three groups) since data did not meet the assumption of Gaussian distribution. Multiple logistic regression models were employed to evaluate the association between seropositivity and patients’ characteristics, including age, sex, COVID-19 severity, and influenza vaccination status. A p-value below 0.05 was deemed statistically significant.
Results
Characteristics of studied patients
The characteristics of the studied individuals are summarized in Table 1. Most of them were less than 50 years old and male and experienced mild COVID-19. There was no difference in age between influenza-vaccinated and unvaccinated groups, although the former had a higher percentage of females and individuals with mild COVID-19 (Table 1).
Table 1
Main characteristics of the studied group
| Parameter | All individuals (N = 1318) | Influenza vaccinated (n = 659) | Influenza unvaccinated (n = 659) | p-value |
|---|---|---|---|---|
| Age, mean ±SD (min-max) | 37.2 ±9.8 (18-83) | 37.7 ±9.9 (18-83) | 36.7 ±9.6 (18-63) | 0.08a |
| < 50 years, % (n) | 88.8 (1170) | 88.2 (581) | 89.4 (589) | 0.47b |
| ≥ 50 years, % (n) | 11.2 (148) | 11.8 (78) | 10.6 (70) | |
| Gender, % (n) | ||||
| Male | 79.7 (1050) | 76.0 (501) | 83.3 (549) | 0.001b |
| Female | 20.3 (268) | 24.0 (158) | 16.7 (110) | |
| COVID-19 severity, % (n) | ||||
| Asymptomatic | 28.0 (369) | 17.3 (114) | 38.7 (255) | < 0.001b |
| Mild | 69.8 (919) | 80.3 (529) | 59.3 (391) | |
| Severe | 2.2 (29) | 2.4 (16) | (13) | |
Seroprevalence and concentration of anti-SARS-CoV-2 IgA antibodies
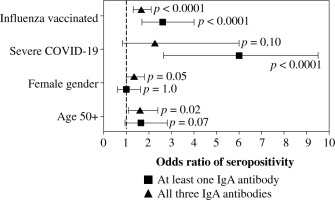
The overall seroprevalence of anti-NP, anti-RBD, and anti-S2 IgA antibodies in the studied group was 32.5%, 58.9%, and 14.3%, respectively. The majority (67.2%) had produced at least one anti-SARS-CoV-2 IgA antibody, but only 8.3% produced all three. As shown in Table 2, seroprevalence differed among the various subgroups. Individuals ≥ 50 years had a higher seroprevalence of anti-NP and anti-RBD, and women produced anti-RBD more frequently, while subjects who were recovering from severe COVID-19 had the highest prevalence of anti-NP and anti-S2 antibodies. Influenza vaccination status was the only factor associated with seroprevalence of all three anti-SARS-CoV-2 IgA antibodies, higher levels of which were observed in individuals vaccinated against influenza in the 2019/2020 epidemic season (Table 2). Moreover, vaccinated individuals were more frequently positive for at least one and all three IgA antibodies, similarly to those who had severe COVID-19 or were aged over 50 years (Table 3).
Table 2
Seroprevalence (%) of anti-SARS-CoV-2 IgA anti-bodies in relation to characteristics of the studied group (N = 1318)
Table 3
Seroprevalence (%) of at least one anti-SARS-CoV-2 IgA antibody and all three determined antibodies (anti-NP, anti-RBD, and anti-S2) in relation to characteristics of the studied group (N = 1318)
Because age, gender, COVID-19 severity, and influenza vaccination status differentiated the seroprevalence of anti-SARS-CoV-2 IgA antibodies, all of them were included in the multivariate analysis. Being vaccinated against influenza in the 2019/2020 infection season was associated with higher odds of seropositivity of all three IgA antibodies. In addition, individuals aged ≥ 50 years had high odds of anti-NP and anti-RBD seroprevalence, while those who were recovering from severe COVID-19 had higher odds of anti-NP and anti-S2 IgA antibodies (Fig. 1). Influenza vaccination was also associated in multivariate analysis with significantly higher odds of being seropositive for at least one of the considered IgA antibodies as well as being seropositive for all three of them (Fig. 2).
Fig. 1
Logistic multiple regression results on the association between seropositivity for IgA antibodies against SARS-CoV-2 nucleocapsid protein (A), receptor-binding domain (B), and S2 subunit of spike protein (C), and characteristics of COVID-19 convalescents (N = 1318). The data are presented as the odds ratio and 95% confidence interval
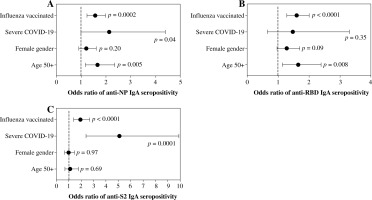
Fig. 2
Logistic multiple regression results on the association between seropositivity for at least one IgA antibody and all three determined IgA antibodies against SARSCoV-2 (anti-NP, anti-RBD, and anti-S2), and characteristics of COVID-19 convalescents (N = 1318). The data are presented as the odds ratio and 95% confidence interval
The serum concentrations (mean ±SD) of anti-NP, anti-RBD, and anti-S2 IgA antibodies in the studied cohort were 448 ±219, 437 ±187, and 322 ±119 U/ml. In most cases, they were not differentiated among particular subgroups, except for higher anti-NP noted in individuals aged less than 50 (Table 4).
Table 4
Serum concentrations of anti-SARS-CoV-2 IgA antibodies (U/ml) in relation to characteristics of the studied group (N = 1318). The data are presented as mean ±SD
Discussion
It is postulated that influenza vaccination, similarly to other vaccines, e.g., Bacillus-Calmette-Guérin (BCG) vaccine or measles, mumps and rubella (MMR) vaccine [25–27], may provide benefits to heterologous viral challenges [14, 28, 29]. These observations mostly concern improved protection against other infections or lower disease severity. The present study shows that such additional benefits of vaccination may also relate to better plasticity of adaptive humoral immunity in response to heterologous infection, i.e., by SARS-CoV-2. Our previous study showed this in relation to IgG antibodies in patients who experienced mild COVID-19 [7], while this study evidences it in relation to IgA class and demonstrates that it is independent of the severity of infection, age, and sex.
There is increasing interest in vaccine-induced trained immunity, a phenomenon of long-term reprogramming of innate cells through epigenetic modifications that ultimately enable non-specific protective effects [30]. In line with this, studies have demonstrated that influenza vaccination is associated with transcriptional and functional reprogramming of innate immune cells, leading to changes in the regulation of inflammatory responses, cell signaling, and antigen presentation [9]. One of the most upregulated genes in monocytes is CTSS, encoding cathepsin S, a protein fundamental for MHC II maturation, processing and presentation of antigens [31]. A similar protein, cathepsin L, is well known to play a role in endocytic cellular entry of SARS-CoV-2 [32]. However, cathepsin S has been shown to cleave spike protein at G700 and V860 to release 17, 46, and 78 kDa fragments [33]. This suggests that increased monocytic expression of CTSS in individuals vaccinated against influenza could represent one of the pathways of enhanced presentation of SARS-CoV-2 antigens, ultimately stimulating better and broader adaptive humoral responses, including the production of specific IgA antibodies shown to dominate the early phase of the adaptive response to SARS-CoV-2 [16, 17].
Further studies are required to confirm the observations reported in the present research under controlled conditions, e.g., using in vivo experimental models with rodents vaccinated against influenza and challenged with SARS-CoV-2. Moreover, broad screening of the epigenetic modifications in isolated populations of immune cells before and after influenza vaccination in animals and humans would enable a better understanding of how it could stimulate heterologous responses of adaptive immunity. These studies are necessary to directly assess whether trained immunity is involved in the phenomenon observed in the present study and, if so, through which pathways. Last but not least, it is of interest to explore whether a similar association as reported in the present study could also occur in relation to other viral infections, e.g., respiratory syncytial virus.
Studies examining humoral responses following COVID-19 vaccination indicate that serum anti-RBD IgA antibodies confer protection against SARS-CoV-2 infection [34]. However, infection-induced IgA antibodies may provide even more potent protection as they can recognize epitopes beyond the spike antigen, e.g., associated with nucleocapsid protein. In line with this, systemic IgA antibodies in COVID-19 were found to contribute to viral neutralization [35] and protect against infection [36]. Therefore, increased IgA seroprevalence associated in the present study with seasonal influenza vaccination may contribute to decreased COVID-19 burden related to reinfections. It would also be interesting to explore whether influenza vaccination is associated with improved production of circulating IgA antibodies against SARS-CoV-2 induced in response to COVID-19 vaccination.
Besides the association of influenza vaccination with a better humoral response, an important observation from the present study is that 33% of studied COVID-19 convalescents had no detectable serum levels of any of the investigated anti-SARS-CoV-2 IgA antibodies, 77.5% were negative for anti-NP, 41% for anti-RBD, and 86% for anti-S2. Analyses of anti-SARS-CoV-2 IgG antibodies conducted within a similar period after infection reported much higher seroprevalence [7, 37, 38]. Although concentrations of serum IgA levels tend to diminish faster than the IgG response, the results of previous studies indicate they should not fall below detection limits within one month after infection in seroconverted individuals [17, 39, 40].
Some studies have indicated that IgA responses during SARS-CoV-2 positively correlate with disease severity, although there is no consensus in this regard [17, 39, 41]. The findings of the present research support this indication, since the highest and broadest seroprevalence of IgA antibodies was found in individuals who were recovering from severe COVID-19. However, their concentrations did not differ from those found in mildly or asymptomatically infected patients. Although females frequently mount better humoral responses than men, including in the context of COVID-19 [42, 43], multivariate analysis performed in the present study did not indicate that it affects the prevalence of anti-SARS-CoV-2 IgA antibodies, for which gender differences were not explored previously. Interestingly, the odds of seropositivity for anti-NP and anti-RBD were also higher in subjects aged 50 and over. Older individuals may have a diminished adaptive immune response and frequently produce lower antibody titers after immunization [44–46]. However, studies conducted in the context of COVID-19 have rarely explored the potential age-dependent differences in IgA production [39]. Never-theless, research conducted before the COVID-19 pandemic indicated that serum IgA responses are maintained despite aging and may even be enhanced, as some older individuals had higher IgA titers [47–49]. The present study confirms that compared to younger subjects, IgA plays a more significant role in adaptive humoral immunity to SARS-CoV-2 in those aged ≥ 50, suggesting the need for more frequent determinations of these antibodies in COVID-19 serological surveys conducted across different age groups.
Strengths of our study include analyzing a relatively large number of serum samples collected in a narrow timeframe from influenza-vaccinated and influenza-unvaccinated individuals after primary exposure to SARS-CoV-2 antigen without a history of COVID-19 vaccination. Moreover, the present study provides valuable data on the seroprevalence of IgA antibodies in Polish individuals infected with SARS-CoV-2 in the early pandemic phase, which, contrary to IgM or IgG antibodies, were not investigated in previous research focusing on patients undergoing infection in 2020 [50, 51]. Study limitations should also be stressed. Firstly, it was not designed to explore whether increased seroprevalence of IgA antibodies associated with influenza vaccination or other parameters translated into improved protection against reinfection or less severe reinfections. As long as such an effect may occur, it would require direct research that at present is lacking. Secondly, the analyzed serum samples were collected prior to the emergence and domination of SARS-CoV-2 variants of concern, including the highly transmissible Omicron lineage, and from individuals vaccinated against influenza in the 2019/2020 epidemic season. Therefore, generalizing the phenomenon reported in the present study should be done cautiously until novel data become available. Thirdly, due to the unavailability of information, the study did not include selected variables that could also influence humoral responses, e.g., using immunosuppressive agents (prior to and during the SARS-CoV-2 infection) and other medications, specific comorbidities, nutritional status, or body mass index. Lastly, serum IgA concentrations determined in the present study do not necessarily correlate with their secretory counterparts, as the local immune responses are typically generated independently from systemic IgA. Mucosal IgA antibodies play a major role in neutralizing respiratory viruses at their entry site; therefore, it would be interesting to determine whether influenza vaccination might also be associated with better heterologous responses at the mucosal site. However, addressing it in naÏve individuals may currently be challenging, since it was expected that by the end of 2023 the vast majority of the world’s population would have been exposed (often multiple times) to SARS-CoV-2 antigen(s) through natural infection and/or vaccination [52, 53]. At the same time, it should be noted that the intramuscular inactivated influenza vaccine (received by the individuals included in the present study) primarily elicits circulating antibodies; thus, its effect on heterologous mucosal IgA responses is unlikely.
Conclusions
The present study provides additional evidence that the benefits of seasonal influenza vaccination may go beyond homologous protection by improving adaptive immune responses to heterologous challenges. As shown, individuals vaccinated against influenza approximately one year prior to SARS-CoV-2 infection had a higher and broader seroprevalence of anti-SARS-CoV-2 IgA antibodies. Further studies are required to elucidate the exact mechanism behind this phenomenon and explore whether it can also occur in the context of adaptive cellular immunity.


