Introduction
Laparoscopic sleeve gastrectomy (SG) is currently the most commonly performed bariatric surgery worldwide and in Poland [1, 2]. The relatively short learning curve, excellent short-term weight loss, and safety profile are components of its success [3–5]. Revisional bariatric surgery (also called re-do bariatric surgery, RBS) has been reported to be necessary in 5–25% of all cases, while in some reports it is as high as 50% of cases [6–8]. RBS is emerging as the third most commonly performed type of bariatric operation [9–11]. Inadequate body weight loss, weight regain, and unsatisfactory control of associated medical conditions are typical reasons for RBS. Thus bariatric surgeons are challenged by an increasing number of SG patients requiring re-do to become a more efficient procedure in the long term. The first consensus on RBS by Mahawar et al. has agreement of options for RBS after SG [12]. Those include Roux-en-Y gastric bypass (RYGB), one anastomosis gastric bypass (a.k.a. mini gastric bypass, OAGB), biliopancreatic diversion/duodenal switch (BPD/DS) and single anastomosis duodeno-ileal bypass after SG (SADI-S). Routinely all of these should be laparoscopic procedures. SADI-S is considered a novel procedure, but is quickly gaining recognition, including as RBS after failed SG.
Aim
We aimed to discuss surgical technique and analyze initial outcomes after introduction of single anastomosis duodeno-ileal bypass after sleeve gastrectomy with 1-year follow-up.
Material and methods
This is a retrospective cohort study of consecutive patients who underwent re-do bariatric surgery – revisional single anastomosis duodeno-ileal bypass – after sleeve gastrectomy between January and December 2021. Inclusion criteria: 18 years old and older patients, weight regain or insufficient weight loss (< 50% excess weight loss) after primary laparoscopic sleeve gastrectomy, eligible for re-do single anastomosis duodeno-ileal bypass after sleeve gastrectomy. The study setting was a secondary referral public hospital. Follow-up was completed in all cases 1 year after surgery.
Surgical technique of primary SG
The technique used for SG was standard. The stomach was completely mobilized along the greater curvature with transection of the short gastric vessels. A 35-Fr bougie was used to trim the diameter of the stomach during gastric longitudinal resection starting 6 cm proximal to the pylorus. The stapler line is routinely reinforced with the running suture.
Preoperative counselling and preparations
Preoperatively at least 12 months of psychological and dietary intervention was attempted. If it failed, patients were considered candidates for RBS. Currently in our department the preferable first choice option for those who failed to achieve sufficient weight loss is single anastomosis duodeno-ileal bypass (SADI-S). Patients underwent evaluation of general medical status as a standard protocol for bariatric surgery. The hospital admission was one day prior to surgery. All patients were administered 40 mg of enoxaparin subcutaneously at 8 p.m. every day including surgery and 40 mg of pantoprazole on the surgery day. Patient care was consistent with ERAS and ERABS guidelines [13–15]. On admission (1 day prior to surgery), every patient was informed about the targeted length of stay of 3 days.
Surgical technique of re-do surgery – SADI-S
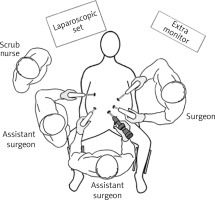
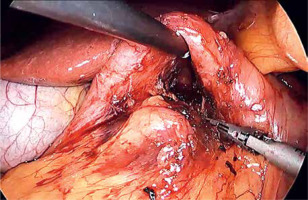
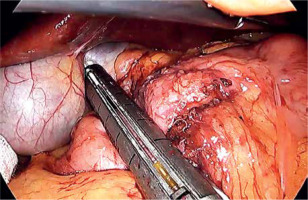
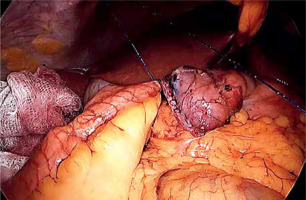
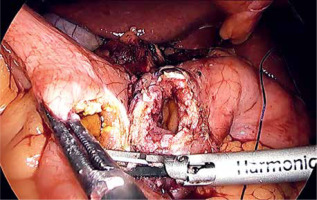
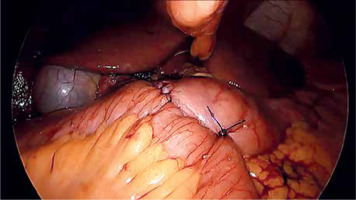
Laparoscopic SADI-S was performed with a five-port technique (Photo 1). Initial access was through a 5–12-mm universal optic trocar in the left midclavicular line 2 cm above the transverse umbilicus plane. Pneumoperitoneum was established to a pressure of 12–15 mm Hg with carbon dioxide. A 10-mm 30° laparoscopic camera was used. Next, a 5–12-mm universal port was introduced above the umbilicus. Nathanson’s liver retractor was introduced below xiphoid process. A 5-mm port was introduced below the left costal margin and the other one in the right midclavicular line 2 cm below the umbilicus. The operating surgeon is standing on the left side of the patient during dissection of the duodenum and between the patient’s legs during anastomosis. The first assistant switches position with the operating surgeon and the second assistant stands on the right side of the patient (Figure 1). The peritoneum and small intestines are inspected for adhesions. Then, the first portion of the duodenum is dissected. We attempt to avoid dissection of the distal stomach, opening of the lesser sac and ligation of the right gastric and gastroepiploic vessels. This helps to maintain optimal vascular supply to the duodenum. Dissection of the duodenum is carried out with a harmonic scalpel. First the superior and then the inferior wall of the duodenum are freed from adhesions and a window is created between the duodenum and pancreas (Photo 2). The duodenum is then transected at least 2–3 cm beyond the pylorus with a 60-mm ECHELON FLEX ENDOPATH stapler with white cartridge (Photo 3). Next, the ileocecal valve is identified and 250 cm of small bowel is measured proximal to the ileocecal valve. End-to-side (duodenum-to-ileum) anastomosis is performed. First the antimesenteric wall of the bowel is sutured to the staple line of the proximal duodenum using a continuous 4/0 barbed polydioxanone suture (Photo 4). The efferent limb was descending on the patient’s right, and the afferent limb was ascending on the left. A duodenotomy and enterotomy approximately 2 cm long is performed with a harmonic scalpel (Photo 5). The hand sutured double layer anastomosis is performed with running 4/0 polydioxanone sutures (Photo 6).
Postoperative course, discharge from hospital and follow-up
Patients were allowed to drink clear liquids just after the procedure. IV fluids were restricted to encourage patients to use oral hydration. Diuresis was monitored for 2 consecutive days. On postoperative day 1, routine upper gastrointestinal fluoroscopy with 60 ml of water-soluble contrast (iohexol) was performed to assess passage of contents through the anastomosis.
Discharge criteria: oral diet tolerance (tolerance of mixed-food diet, drinking at least 1000 ml of fluids), no need for intravenous drugs or fluids, balanced diuresis, physical activity at a level similar to pre-surgery time. All patients attended a follow-up visit with the operating surgeon 2 weeks after surgery. The patients were all instructed about postoperative vitamin supplementation and a meeting was planned with a bariatric dietician one month after surgery (the further plan was individual). A clinical psychologist met patients 6 months after surgery or more frequently if needed. Postoperative follow-up visits were set at 6 and 12 months after surgery.
Statistical analysis
Data were analyzed with Statistica 13.3 PL (TIBCO Software, Palo Alto, USA). Continuous data are presented as medians with first and third quartiles (Q1-Q3). Repeated measurements of body mass weight (BMI), percentage weight loss (%TWL) and percentage excess body mass index loss (%EBMIL) were compared using Friedman’s ANOVA.
Ethics
All procedures have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Informed consent for surgical treatment was obtained from all patients before surgery. This type of study did not require formal consent of a bioethics committee.
Results
Fourteen consecutive patients underwent SADI-S as a revisional procedure after failed sleeve resection and completed 1-year follow-up. This group consisted of 6 (43%) males and 8 (57%) females. Median age of patients was 45 (41–53) years. Details of patients’ characteristics are presented in Table I.
Table I
General characteristics
Median operative time was 120 (85–125) min. Median length of hospital stay was 4 (3–6) days. No perioperative morbidity was recorded. Four (28%) patients reported recurrent abdominal crampy pain and diarrhea that required dietary advisement and pharmacological therapy in the postoperative period. No reoperations, mortality or readmissions were recorded during 1-year follow-up. Bariatric results are presented in Table II.
Table II
Follow-up
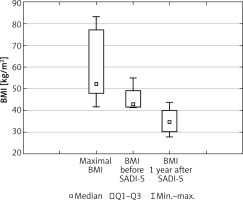
Changes in patients median BMI during the study are presented in Figure 2. As shown, SADI-S resulted in further weight loss, resulting in median BMI of 37.55 (36.29–39.43) kg/m2 1 year after SADI-S. The observed additional %TWL 1 year after SADI-S was 18.65% (17.25–21.89%), while additional %EBMIL was 35.88% (29.18–41.92%). There was 1 case of diabetes mellitus type 2 remission and improvement in glycemic control in 1 patient. 4/6 patients (66.67%) had improvement in control of hypertension. Regretfully there were no remissions. There were no other changes in comorbidities 1 year after procedures.
Discussion
The single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) was designed as a simplification of the biliopancreatic diversion/duodenal switch in an attempt to decrease operating time and postoperative morbidity, while maintaining its principles and efficacy. Comparison of 220 patients after BPD-DS and 240 after SADI-S was performed in a study by Lind et al. [16]. Mean LOS was comparable (3.5 and 3.1 days for BPD-DS and SADI-S). Operative time was shorter in the SADI-S group (141 ±57 min, 167 ±34 min). The mean %EWL and %TBWL was comparable between groups at 6, 12, and 24 months respectively. Overall complication rates were also similar, 14% after BPD-DS and 18% after SADI-S. SADI-S had similar readmission, reoperation rates and mortality (BPD-DS 0.9% vs. SADI-S 0.4%) [16]. This study was one of the largest single-center comparative studies between these two procedures. Moreover, SADI-S is aimed at better control of nutritional status and it was hoped to decrease the risk of late malnutrition [17]. Patients after BPD-DS had lower levels of vitamin B12, iron, vitamin E, and zinc than after SADI-S in long-term observations. It is also believed that revision after SADI-S (if needed) is less demanding than BPD-DS. However, there is a lack of exact data concerning this subject [18–20].
Although results of primary SADI-S are known, data regarding its efficacy as a revisional procedure after failed SG remain scarce [21–23]. SADI as a revisional procedure after SG is effective in terms of weight loss in the present series, leading to an additional %TWL of 18.65% at 12 months (data referring to 14 patients), i.e. additional %EBMIL of 35.88%. Similar results were reported in other cohort studies [24, 25]. A review of literature revealed evidence of a slight advantage of SADI-S over OAGB-MGB after SG [18, 21, 26–31]. Multiple studies have reported superiority of SADI-S when compared to RYGB after SG in terms of achieving extra weight loss [26, 30, 32].
In our series no perioperative morbidity was recorded. Some patients experienced recurrent abdominal crampy pain and diarrhea that required dietary advisement and pharmacological therapy in the postoperative period. No reoperations, mortality or readmissions were recorded during 1-year follow-up. Surve et al. analyzed one of the largest SADI-S cohorts with long-term follow-up, including the results of 750 primary SADI-S [33]. The short-term complication rate was 7.8%. The most common short-term complications that occurred were nausea and vomiting and wound infection. During the first 30 days, 0.9% experienced Clavien-Dindo [34] grade III b complications, while the short-term mortality rate was 0.1%. The 30-day readmission and reoperation rates were 1.1% and 1.1%, respectively. The most common reasons for 30-day readmission and reoperation were nausea and vomiting, and intraabdominal hematoma, respectively. The long-term complication rate was 11.7% (> 30 days); the most common were diarrhea, nausea and vomiting and strictures. 4.4% experienced Clavien-Dindo grade III b complications. The long-term mortality rate was 0.4%. Similar data were provided by smaller cohorts of non-primary SADI-S (after SG), as presented in works by Bashah et al. and Liagre et al. [24, 25].
Concerning the surgical technique, SADI after SG may or may not include a re-sleeve of the gastric tube. Re-sleeve is associated with a higher rate of postoperative leaks, which is estimated to be around 2% [35]. Re-sleeve was not routinely done in our series.
Our study demonstrated a positive effect on improving the comorbidities (diabetes mellitus type 2 an hypertension) over 1 year. Prior studies showed that SADI-S was associated with a significant potential improvement of comorbidities when compared to other revisional procedures [17, 25, 36].
The results of our initial experience are promising. With this publication we would like to present the technical details for further comparability of surgical technique. Considering emerging evidence for recommendation of SADI-S as revisional bariatric surgery after failed primary sleeve gastrectomy, SADI-S may become the procedure of choice in most cases.
Conclusions
SADI-S is promising re-do surgery for insufficient weight loss or weight regain after laparoscopic sleeve gastrectomy with low postoperative morbidity. Observed additional %TWL 1 year after SADI-S can be expected at a level of ~19%, and additional %EBMIL of ~36%, with significant improvement of obesity-related comorbidities.