Introduction
The causes of diabetes in both chronic pancreatitis (CP) and pancreatic ductal adenocarcinoma (PDAC) have not yet been fully explained. Carbohydrate metabolism disorders may result from absolute insulin deficiency and peripheral insulin resistance [1]. Most of the patients with pancreatic diabetes come from the group diagnosed with CP (76%). The second most frequent reason for this type of diabetes is PDAC (9%) [1, 2]. Due to the lack of sensitive and specific markers to distinguish CP from PDAC in clinically difficult cases, it is advisable to look for new markers that differentiate these two diseases. The detection of a marker indicating PDAC at an early stage of its development based on the coexistence of pancreatic diabetes would be a breakthrough.
In patients with CP, the dynamics of the endocrine disorders depend on the aetiology and duration of the disease. There is a convergence between exo- and endocrine disorders [2, 3]. In alcoholic CP, endocrine failure usually occurs in the advanced stage of the disease, after about 8–10 years [3]. The development of endocrine disorders in the course of CP results from a decrease in insulin secretion, caused by progressive fibrosis of the organ, damage and loss of α- and β-cells of pancreatic islets [4]. In patients with CP, progressive insulin resistance also occurs [5]. In the pathogenesis of diabetes development there is also a malfunction of incretin hormones, disturbances of microcirculation and impaired hepatic gluconeogenesis inhibition by insulin [6].
Data confirming the relationship between long-standing type 2 diabetes and the increased risk of PDAC come from numerous epidemiological studies [7]. The phenomenon of insulin resistance and hyperinsulinaemia probably results in increased pancreatic islet proliferation, which may predispose to the development of cancer [8]. On the other hand, it has been proven that newly detected diabetes after the age of 50 years significantly indicates recognition of PDAC [7]. One of the likely signals to start carcinogenesis is a change in the differentiation and function of pancreatic islet cells [8]. The participation of these cells in the neoplastic process is also confirmed by the presence of carbohydrate metabolism disorders in the case of small tumours located on the periphery of the organ. Moreover, in the development of diabetes in the course of PDAC, the participation of diabetogenic proteins synthesised by the tumour and progressive insulin resistance is mentioned [8, 9].
Lots of studies show close links between the IGF axis and glucose metabolism, including pancreatic diseases [10]. The insulin-like growth factor (IGF) axis consist of two growth factors, IGF-1 and IGF-2, in addition to several IGF binding proteins (IGFBP-1 to IGFBP-6), which work together to regulate the amount of free IGF-1 and IGF-2 in serum [11]. IGFs have structural homology to insulin, playing an important role in proliferation and differentiation of normal and malignant cells [11, 12]. The IGFs bind to three cell-surface transmembrane receptors: IGF-1R (type 1 IGF receptor), IGF-2R (type 2 IGF receptor), and IR (insulin receptor) [12].
IGF-1 has a wide variety of effects, but, essentially, these can be divided into acute metabolic effects and long-term growth-promoting effects [11]. The acute actions of IGF-1 overlap with those of insulin on carbohydrate and protein metabolism to promote energy storage. They include stimulation of amino acid uptake into skeletal muscle and stimulation of peripheral glucose uptake. Its long-term effects are on cell proliferation, differentiation, and anti-apoptosis [13]. IGF-1 plays a role in the regulation of β-cell mass and the regulation of insulin secretion and sensitivity [14]. Insulin could stimulate hepatic IGF-1 synthesis and suppress hepatic IGFBP-1 synthesis in the liver, which could lead to an increase in the serum concentration of IGF-1 [15]. IGF-1 and the IGF-1 receptors are highly expressed on the surface of pancreatic cancer cell lines, which initiate intracellular signalling transduction associated with proliferation, invasion, and expression of mediators of angiogenesis [16]. Numerous studies have shown a relationship between IGF-1 levels, body fat, and peripheral insulin resistance [17]. Adipose tissue is one of the sources of IGF-1 production, and the peripheral insulin resistance occurring in the course of obesity affects the concentration of this protein [18].
The data highlights the importance of local IGF-1 production in regulating cell differentiation/proliferation and supports the concept that tissue-derived IGF binding proteins may represent a potential substrate capable of modifying autocrine/paracrine IGF-1 effects [19]. Quantification of the corresponding proteins in plasma might be useful for PDAC diagnosis. IGFBP-2 has the ability to discriminate PDAC patients at an early stage from healthy controls, and it appeared to be increased in diseases posing a risk of pancreatic malignancy [20]. There is abundant evidence that IGFBP-2 plays a role in the promotion of various cancers [21]. The interaction of the Arg-Gly-Asp motif in IGFBP-2 with integrins typically results in stimulatory effects towards cancer cells [22]. Moreover, nuclear transport of IGFBP-2 is reported to be associated with a tumourigenic effect by promoting angiogenesis through activation of VEGF transcription [23].
Aim
The aim of this study was to evaluate the serum concentration levels of IGF-1 and IGFBP-2 in patients with CP and newly diagnosed PDAC. Their values in patients with CP, PDAC, and concomitant diabetes mellitus (DM) were also assessed.
Material and methods
The study included 83 patients with CP, 92 patients with PDAC diagnosed within the last 6 months, and 20 healthy controls. The study included 83 patients with CP – 62 men and 21 women. The mean age of the men was 29–79 years (46.68 ±12.60 years), and the women 26–78 years (51.23 ±15.18 years). Ninety-two patients had PDAC diagnosed within the last 6 months – 49 men and 43 women, men aged 36–94 years (66.56 ±10.90 years), women aged 41–86 years (64.51 ±9.98 years), and 20 controls – 12 men and 8 women, aged 37–63 years (48.4 ±12.56 years).
The diagnosis of CP was based on the clinical picture and imaging (ultrasonography, computed tomography, endosonography), according to the Cambridge criteria. The diagnosis of CP required visualisation of the following abnormalities in imaging tests: parenchymal/ductal calcifications, dilatation and/or irregularities in the walls of the main pancreatic duct or secondary ducts. CP on alcohol aetiology was diagnosed when consuming alcohol in the amount of 60 g a day during the last 2 years and after excluding other toxic, metabolic, and barrier factors. PDAC was diagnosed with at least two imaging techniques and confirmed with a histopathology of a surgical specimen or endosonography with fine needle aspirate cytology (EUS-FNA).
The blood samples were collected from each patient with CP, PDAC, and the control group after overnight fasting. The concentrations of IGF-1 and IGFBP-2 were measured in serum with ELISA (Corgenix UK Ltd, R&D Systems).
The study was undertaken under strict ethical guidelines, and every participant signed informed consent to document their clinical data. The study was approved by the Ethics Committee of the Medical University of Lodz under the reference number RNN/108/15/KE on 21 April 2015.
Statistical analysis
Introductory statistical analysis of the data used parametric and non-parametric tests. The Shapiro-Wilk test was used to determine whether the data were normally distributed. In such a case Student’s t-test and the Cochrane-Cox test were used in order to assess the hypothesis of equality of means between samples. In the case of data with abnormal distribution, the U Mann-Whitney test was used to determine the equality of means between samples. A p-value of < 0.05 was considered statistically significant. Data presented in the text are expressed as mean ± standard deviation (S.D).
Results
Mean body mass index (BMI) was 22.58 ±5.31 kg/m2 in CP, 23.73 ±6.58 kg/m2 in PDAC, and 24.93 ±4.14 kg/m2 in the control group. DM was diagnosed in 27 patients with CP (32.5%) similarly to the PDAC group with 30 subjects with DM (32.6%).
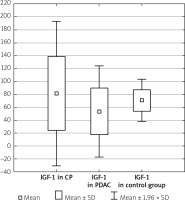
The serum IGF-1 level was significantly higher in the CP group compared with the PDAC group (81.11 ±57.18 ng/ml vs. 53.18 ±36.05 ng/ml, p < 0.001), and both CP and PDAC patients differed from controls (81.11 ±57.18 ng/ml vs. 70.66 ±16.57 ng/ml, p < 0.001 and 53.18 ±36.05 ng/ml vs. 70.66 ±16.57 ng/ml, p < 0.001) (Table I, Figure 1).
Table I
IGF-1 and IGFBP-2 levels in the CP, PDAC, and control groups
| Parameter studied | 83 CP | 92 PDAC | 20 Control group | P-value |
|---|---|---|---|---|
| IGF-1 [ng/ml] | 81.11 ±57.18 | 53.18 ±36.05 | 70.66 ±16.57 | < 0.001 |
| IGFBP-2 [ng/ml] | 512.42 ±299.77 | 301.59 ±190.36 | 51.92 ±29.40 | < 0.001 |
Figure 1
IGF-1 level in the CP, PDAC, and control groups
CP – chronic pancreatitis, PDAC – pancreatic adenocarcinoma,
IGF-1 – insulin-like growth factor 1.
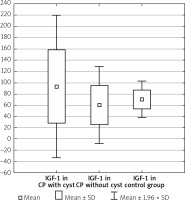
Men with CP compared to women with CP had the IGF-1 levels such as 85.03 ±62.16 ng/ml vs. 69.54 ±37.87 ng/ml, p = 0.489. In the group with CP without DM the IGF-1 level was 91.13 ±65.48 ng/ml vs. 62.20 ±32.38 ng/ml in the group with CP and DM, p = 0.077. The duration of CP did not influence the IGF-1 level (71.41 ±36.84 ng/ml in short-lasting CP up to 8 years of the disease vs. 81.45 ±48.85 ng/ml in long-lasting CP over 8 years, p = 0.462). The presence of calcification did not affect the IGF-1 concentration (84.08 ±73.22 ng/ml without calcification vs. 76.15 ±50.71 ng/ml with calcification, p = 0.867). In CP without distinction of pancreatic ducts, the IGF-1 compared to CP with distinction of pancreatic ducts was (95.30 ±78.59 ng/ml vs. 75.60 ±45.75 ng/ml, p = 0.660). In the group with CP without narrow main pancreatic duct, the IGF-1 was 91.82 ±71.07 ng/ml vs. 72.09 ±54.29 ng/ml in the group with CP and narrow main pancreatic duct, p = 0.475. Patients who had CP without cysts were noted to have a significantly lower level of IGF-1 compared to those with both CP and cysts (60.35 ±34.68 ng/ml vs. 93.55 ±64.78 ng/ml, p < 0.05) (Table II, Figure 2).
Table II
IGF-1 level in CP patients depending on occurrence of cysts
| Parameter | Cysts (–) | Cysts (+) | P-value |
|---|---|---|---|
| IGF-1 [ng/ml] | 60.35 ±34.68 | 93.55 ±64.78 | < 0.05 |
Figure 2
IGF-1 level in CP depending on occurrence of cysts
CP – chronic pancreatitis, IGF-1 – insulin-like growth factor 1.
In CP without DM, IGF-1 was higher compared to those with PDAC without DM (91.13 ±65.48 ng/ml vs. 54.75 ±40.41 ng/ml, p < 0.001). In patients with CP and DM the IGF-1 was also higher in comparison to patients with PDAC and DM (62.20 ±32.38 ng/ml vs. 48.45 ±24.88 ng/ml, p < 0.05) (Table III).
Table III
IGF-1 (ng/ml) level in CP and PDAC patients depending on diabetes
| Diabetes mellitus | 83 CP | 92 PDAC | P-value |
|---|---|---|---|
| (–) | 91.13 ±65.48 | 54.75 ±40.41 | < 0.001 |
| (+) | 62.20 ±32.38 | 48.45 ±24.88 | < 0.05 |
The IGF-1 serum level in men with PDAC was similar to that in women with PDAC (52.93 ±33.72 ng/ml vs. 53.47 ±38.94 ng/ml, p = 0.882). In the group with PDAC without DM the IGF-1 as compared with those with PDAC and DM was (54.75 ±40.41 ng/ml vs. 48.45 ±24.88 ng/ml, p = 0.514). In the group with PDAC and new-onset diabetes the IGF-1 level was 47.10 ±19.53 ng/ml compared to 57.43 ±36.28 ng/ml in patients with PDAC and longstanding DM, p = 0.35. There was also no statistically significant difference in the PDAC group without and with DM in the subgroup: new-onset DM and longstanding DM, respectively (54.75 ±40.41 ng/ml in DM vs. 47.10 ±19.53 ng/ml in new-onset DM, p = 0.919 and 54.75 ±40.41 ng/ml in DM vs. 57.43 ±36.28 ng/ml in longstanding DM, p = 0.954).
The serum IGFBP-2 level was significantly higher in CP patients compared to PDAC patients (512.42 ±299.77 ng/ml vs. 301.59 ±190.36 ng/ml, p < 0.001). In the CP and PDAC group the IGFBP-2 serum level was significantly elevated compared to the control group (512.42 ±299.77 ng/ml vs. 51.92 ±29.40 ng/ml, p < 0.001 and 301.59 ±190.36 ng/ml vs. 51.92 ±29.40 ng/ml, p < 0.001) (Table I, Figure 3). IGFBP-2 in CP without DM was also higher compared to those with PDAC and without DM (559.39 ±281.43 vs. 296.53 ±196.93, p < 0.001) (Table IV).
Figure 3
IGFBP-2 level in the CP, PDAC, and control groups
CP – chronic pancreatitis, PDAC – pancreatic adenocarcinoma,
IGFBP-2 – insulin-like growth factor binding protein 2.
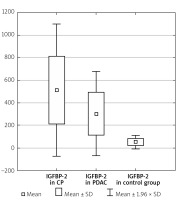
Table IV
IGFBP-2 (ng/ml) level in CP and PDAC patients without diabetes
| Diabetes mellitus | 83 CP | 92 PDAC | P-value |
|---|---|---|---|
| (–) | 559.39 ±281.43 | 296.53 ±196.93 | < 0.001 |
IGFBP-2 in men with CP was 534.54 ±288.76 ng/ml vs. women with CP 442.18 ±331.74 ng/ml, p = 0.329. In CP without DM, the IGBP-2 level was 559.39 ±281.43 ng/ml vs. 504.90 ±330.90 ng/ml, in those with CP and DM, p = 0.776. In patients with short time of CP duration, the IGFBP-2 was 447.28 ±308.13 ng/ml vs. 299.70 ±225.69 ng/ml, in the group with long duration of CP, p = 0.235. In CP without calcification the IGFBP-2 was 615.59 ±226.24 ng/ml vs. patients with CP and calcifications 551.55 ±303.08 ng/ml, p = 0.433. In the group with CP and normal pancreatic ducts the IGFBP-2 was 588.25 ±274.05 ng/ml vs. 531.61 ±309.02 ng/ml among those with extended pancreatic ducts, p = 0.508. In CP without narrow main pancreatic ducts, IGFBP-2 was 637.35 ±259.05 ng/ml vs. 712.00 ±197.70 ng/ml in CP with constricted main pancreatic duct, p = 0.371. Patients with CP without cysts did not differ from those with both CP and cysts (563.52 ±276.24 ng/ml vs. 540.94 ±298.94 ng/ml, p = 0.767).
IGFBP-2 in men with PDAC was 284.44 ±167.44 ng/ml, and in women with PDAC the IGFBP-2 was 320.79 ±213.56 ng/ml, p = 0.767. In the PDAC group without DM, the IGFBP-2 was 296.53 ±196.93 ng/ml in comparison to PDAC and DM 312.57 ±183.70, p = 0.327. Between new-onset DM vs. longstanding DM in the course of PDAC, the IGFBP-2 level was 283.89 ±190.97 ng/ml vs. 375.98 ±187.78, p = 0.173. The IGFBP-2 level did not depend on whether there was no DM or DM was new-onset during PDAC (296.53 ±196.93 ng/ml vs. 283.89 ±190.97 ng/ml, p = 0.940). IGFBP-2 in PDAC without DM did not differ from IGBP-2 in PDAC with longstanding DM (296.53 ±196.93 ng/ml vs. 375.98 ±187.78, p = 0.071).
Discussion
There are a relatively small number of publications assessing both IGF-1 and IGFBP-2 levels among patients with CP and PDAC. It is extremely important to look for new biomarkers that enable the differentiation of PDAC and endocrine disorders which might be distinguished as one of as the first symptom of PDAC.
Pancreatic diabetes accounts for only 5–10% of all cases of DM [24]. However, new-onset diabetes caused by the cancer appears to be a clinically useful marker of asymptomatic PDAC [25]. In many PDAC patients, onset of DM occurs when they would have no cancer-related symptoms. Thus, new-onset DM is the only clue to the presence of asymptomatic PADC. The high prevalence of diabetes in small tumours < 2 cm, early stage tumours, and the onset of diabetes nearly 2 years before diagnosis and prior to radiologically detectable tumour tends to favour a humoral process rather than a tumour local effect [26]. These data suggest that new-onset DM in pancreatic cancer is probably induced by the tumour, which is not likely to be explained simply by tumour-induced gland destruction. In our opinion, IGF-1 may be an indicator that signals whether pancreatic diabetes is from CP or PDAC. Our results show that diabetes does not have an influence on IGF-1 levels in PDAC patients. The IGF axis, especially IGF-1, may be a factor closely related to the onset of PDAC due to its impact on glucose metabolism. Our outcome can be explained by the ability of insulin to activate IGF-1 receptors through hyperinsulinaemia and insulin resistance [27]. According to the study by Basso et al., IGF-1 could be involved in influencing glucose homeostasis [28]. In another study, no correlation was found between IGF-1 and tumour size or stage, but its level was inversely correlated with fasting serum glucose levels (p < 0.05) [29].
Elevated levels of IGF-1 are observed in colorectal, prostate, lung, and breast cancer [26, 30]. In our study the PDAC patients were characterised by a lower IGF-1 serum level. Some epidemiological studies have shown that a high concentration of IGF-1 is observed in CP and PDAC, more so than in the controls, but others did not confirm that finding [28, 29]. States in which insulin is markedly elevated are associated with lower serum IGF-1 concentrations [29]. As PDAC is characterised by insulin resistance and increased insulin levels, this could explain the reduced level of IGF-1 among our patients.
We indicate that IGF-1 and IGFBP-2 are good biomarkers in recognising pancreatic diseases. Moreover IGF-1 may be an indicator that signals whether pancreatic diabetes comes from CP or PDAC, and it may indicate the presence of a pancreatic cyst in CP patients. Karna et al. suggested that disturbances in tissue collagen metabolism during pancreatic diseases may result from deregulation of IGF-1 homeostasis. This could be an explanation for our differing results, because we know that IGF-1 stimulates collagen biosynthesis through interaction with IGF-1 receptor. Moreover, IGFBPs regulate the activity of IGF-1 [30, 31].
The level of IGFBP-2 is reported to be increased in PDAC patients’ serum, in accordance with our results [29, 32]. According to other authors, the study shows a 2.5-fold increase in serum levels of IGFBP-1 in patients with CP and about a six-fold increase in serum of patients with PDAC [31]. In our results, IGFBP-2 levels were 9.9-fold increased in the CP group compared to controls, and 5.8-fold in PDAC patients. In the study by Yoneyama et al., the results revealed that IGFBP-2 and IGFBP-3 have the ability to discriminate PDAC patients at an early stage from healthy controls, and IGFBP-2 appeared to show an increased risk in diseases of pancreatic malignancy. Furthermore, diagnosis of PDAC using the combination of carbohydrate antigen 19-9 (CA19-9), IGFBP-2, and IGFBP-3 is significantly more effective than CA19-9 alone. Early diagnosis with this marker combination may improve the prognosis of PDAC patients [20].
We show that the IGFBP-2 level is lower when compared to patients with CP. Diabetes co-occurrence did not influence IGFBP-2 levels in our PDAC group. Some research reports that the level of IGFBP-2 increased slightly as the clinical stage became more advanced. IGFBP-2 should preferably be released/induced/produced by the cancer and should correlate with tumour burden, increasing with tumour growth [20, 32]. In the collaborative meta-analysis by Gong et al. the results showed for the first time that serum IGF-1, IGF-2, IGFBP-1, and IGFBP-3 concentrations as well as the IGF-1/IGFBP-3 ratio were not associated with risk of PDAC. Sub-group analysis also did not show any significant associations [15].
In our study, the mean age of patients diagnosed with PDAC was higher compared to the CP group and was about 66 years, which could have an effect on lowering the level of IGF-1 level. Numerous studies observed that as the body ages, the levels of IGF-1 and IGFBP-3 decrease [33]. Among patients over 65 years of age, low IGFBP-1 levels are associated with an increased incidence of glucose tolerance disorders [34]. In the presence of carbohydrate disorders, IGF-1 and IGFBP-1 levels are lower among middle-aged patients. There are no statistically significant differences in IGF-1 among older patients, which may be due to progressive metabolism disorders [33]. Lowering the previously increased result in CP could be an indicator of the development of PDAC against the background of CP.
Conclusions
It is extremely important to search for PDAC biomarkers that enable detection of cancer at an early stage of its development. However, it should be taken into account that biochemical tests can only have a complementary and auxiliary meaning compared to imaging tests. Due to the frequent occurrence of diabetes in the course of PDAC, the detection of a protein that differentiates pancreatic diabetes from other endocrine disorders may be crucial. In our study IGF-1 and IGFBP-2 are good biomarkers of pancreatic diseases, especially CP and PDAC. IGF-1 may be an indicator that signals whether pancreatic diabetes is from CP or PDAC. Non-specific biomarkers, regardless of their value, even more than specific biomarkers, can only have complementary significance in the context of other results, especially visualising ones, and they cannot replace full diagnostics in accordance with current recommendations.










