Psoriasis is an incurable, chronic auto-inflammatory disease, which is prevalent worldwide. It is clinically heterogenous, with subtypes such as psoriasis vulgaris, inverse, guttate, pustular, and erythrodermic, of which psoriasis vulgaris is the most common. Pathogenesis of the disorder is multifactorial, encompassing genetic predispositions, environmental factors, and dysfunction of the adaptive and innate immune system within the context of skin, which puts psoriasis among autoimmune disorders [1]. Contemporary advancements in the understanding of its pathogenetic mechanisms have offered a more detailed picture of the disease’s intricate immunological dynamics. We now recognize the pivotal role that cytokines play in mediating the aberrant immune responses, which enables a more personalized approach to treatment with targeted therapies designed to modulate specific interleukins. Currently, there are four distinct classes of biologics available for treating psoriasis, each targeting specific cytokines involved in the disease process. These include anti-TNF-α (e.g., adalimumab, infliximab), anti-IL-17 (e.g., secukinumab), anti-IL-12p40 or IL-23p40 (ustekinumab), and anti-IL-23p19 (e.g., tildrakizumab) agents. In total, there are 11 biologic agents within these classes [2]. These therapies exert either suppressive or stimulatory effects on crucial pathogenetic pathways, offering a diverse range of treatment options that can be tailored to each patient.
In this article, we aim to illuminate the roles of interleukin-30 (IL-30) and interleukin-27 (IL-27) in inflammation and autoimmune diseases, with a specific focus on psoriasis. The exploration of IL-30’s function in these contexts has been relatively underrepresented in past literature, although it is an intriguing frontier of investigation.
Interleukin 30 is a p28 subunit of interleukin 27, which is formed in combination with Epstein-Barr virus-induced gene 3 [3]. The properties of IL-30 cannot be analysed in isolation from IL-27 as the two coexist in vivo and their actions are largely antagonistic. Furthermore, the available methods for studying the concentration of IL-30 are not sufficiently selective, thus research findings pertaining to the autonomous functions of IL-30 ultimately involve the IL-30/Ebi3 complex [4].
The actions of IL-27 in the context of inflammatory responses have been extensively investigated. It exerts its effects on FOXp3+ regulatory T (Treg) cells, promoting their ability to suppress autoimmune inflammation [5]. This mechanism is thought to be responsible for hindering the development of experimental autoimmune encephalomyelitis (EAE) after systemic administration of IL-27 [4]. Additionally, IL-27 serves as a potent inducer of IL-10 in T-cells which acts as a safeguard against excessive response [6]. IL-27 inhibits the differentiation of Th2 and Th17 cells while supporting Th1 cell differentiation [4]. IL-30 is thought to antagonize gp130 signalling, therefore inhibiting the function of crucial interleukins, such as IL-6 and IL-27 [7].
IL-27 exhibits the capacity to activate natural killer (NK) cells and stimulate the production of interferon-γ (IFN-γ) through Th1 cells, therefore playing a role in bacterial and viral infections [4]. This diverse range of anti-inflammatory mechanisms translates to clinical significance of IL-27 in various inflammatory diseases. IL-30, on the other hand, inhibits cellular and humoral responses in the setting of a parasitic infection through limiting the ability to produce immunoglobulins and altering T-cell responses [8, 9]. IL-27 also counteracts allergic inflammation as its intranasal administration reduces eosinophil infiltration in the airways, mitigates airway hyperresponsiveness, and alleviates allergic rhinitis symptoms [10].
In the context of cancer development, IL-27 exhibits both antitumor and protumor properties [11]. The activity of IL-30 is thought to be largely protumerogenic, based on its ability to influence the immunological profile of cancer cells. In breast cancer studies the administration of IL-30 supported tumour growth and angiogenesis through upregulation of expression of protooncogenes and growth factors, such as VEGF-A and EGF [12]. The roles of IL-27 and IL-30 in inflammation are summarized in Table 1 [4–15].
Table 1
The roles of IL-27 and IL-30 in inflammation
| IL-27 | IL-30 |
|---|---|
|
In psoriasis, the role of IL-27 is still not fully understood. It exerts a pleiotropic effect on the differentiation and responses of T-cells. A majority of studies have found that its expression is upregulated in the skin and serum of patients with psoriasis [16]. The interpretation of IL-27 upregulation remains controversial as it is unclear whether it demonstrates a proinflammatory role in activating Th1 cells or a regulatory mechanism to hinder Th17 cell activity [17]. It is hypothesized that IL-27 promotes the onset of psoriasis, but in the presence of TNF-α it limits the disease [17].
The results of studies conducted to date are often contradicting. In 2013 Shibata et al. found enhanced expression of Th1 cytokines and TNF-a along with the exacerbation of the disease after the injection of IL-27 in mice with imiquimod-induced psoriasis. After administering anti-IL-27 antibody, they observed a downregulated expression of proinflammatory cytokines together with clinical and histological improvement. Expression of Th17 cytokines was not altered in this study [18]. However, in 2017 Chen et al. performed an analogous investigation, administering IL-27 to imiquimod-treated mice, and noticed clinical improvement. As an antibody, they used anti-IL-27p28, which exaggerated the severity of the disease. Moreover, they performed the analysis of IL-27 expression levels in the skin and serum of patients with psoriasis and found them to be significantly reduced compared with healthy control subjects. Extended research showed that administration of IL-27 repressed IL-17 secretion from CD4+ T lymphocytes and anti-IL-27p28 increased IL-17A levels in the serum and skin [19]. The discrepancy may result from differences in methodology and disparities in biology of interleukin secretion between mice and men. The most recent study of cytokine pathways in patients with psoriasis, performed by Michalak-Stoma et al., showed decreased IL-27 levels in the patients’ serum, which was consistent with Chen’s results [20]. Through the evaluation of the concentrations of 18 specific cytokines in the serum of 52 males with psoriasis, they observed a positive correlation between the levels of IL-12 and IL-18 with the Psoriasis Area and Severity Index (PASI). Additionally, a negative correlation was identified between the levels of IL-12 and IL-23 and the duration of the disease, as well as between IL-6 and IL-9 levels and the Nail Psoriasis Severity Index.
Few studies have been carried out regarding the involvement of IL-30 in psoriasis. Four of them found increased expression of IL-30 in the serum of patients with psoriasis compared to healthy controls [8, 21, 22]. In the newest study by Atta, upregulated expression displayed a significant positive correlation with the PASI score [22]. Research performed by Liu et al. focused on the in vivo effect of administered IL-30 on murine models with psoriatic-like lesions induced with imiquimod and Krt14-Vegfa [23]. IL-30 significantly alleviated psoriatic lesions in phenotypical and histopathological examinations, and lowered the expression of ICAM and VCAM. Moreover, the administration of IL-30 reduced IL-23 and IL-17 mRNA without altering IFN-g, which points to its mechanism of action being related to Th17 rather than Th1 cells. At the same time, however, IFN-g, one of the key cytokines in the pathogenesis of psoriasis has been shown to induce the expression of IL-30 mRNA and the production of IL-27 [24].
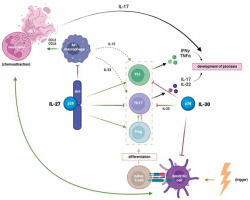
Figure 1 illustrates the roles of IL-27 and IL-30 in the pathogenesis of psoriasis. As can be seen, the interplay of T-cells, dendritic cells and neutrophils, mediated and modulated by cytokines, activates the immune system and contributes to the development of psoriatic lesions. Dendritic cells are stimulated by triggers, such as pathogens, drugs, toxins and stress to produce proinflammatory cytokines, including IL-23, IL-12 and IFN-a, which promote the differentiation of naïve T-cells. TNF-a, produced by DCs, macrophages and T-cells, activates DCs through the IL-23/Th17 axis, creating a positive feedback loop [25]. Activated macrophages attract neutrophils, which form Neutrophil Extracellular Traps (NETs) secreting IL-17 and other proinflammatory mediators [26]. The interaction between NETs and dendritic cells perpetuates chronic inflammation. As mentioned before, IL-27 promotes the differentiation of Th1 cells, inhibits the differentiation of Th17 cells, and hinders the polarization of macrophages into proinflammatory M2. Some studies indicate on the other hand that IL-30 has the potential to independently antagonize Th1 and Th17 cells, which are crucial in the pathogenesis and progression of autoimmune diseases [8]. In vitro, Liu et al. found that IL-30 attenuated the inflammatory response in keratinocytes and suppressed the maturation of dendritic cells, hindering the proliferation of T-cells [23].
IL-30 presents the ability to combine with subunits alternate from Ebi3 and to perform different biological functions. In combination with cytokine-like factor 1 (CLF1), IL-30 was found to induce Th17 differentiation, leading to upregulated IL-17 production [27]. IL-30/CLF1 complex also induced plasma cell differentiation [28].
Liu et al. used the Gene Expression Omnibus database to conclude that expression of IL-30 was significantly higher in the lesional skin of patients with psoriasis compared to samples from non-lesional areas and from healthy volunteers [23]. These findings need to be interpreted with regard to results from other studies on autoimmune diseases, which repeatedly found overexpression of IL-30 protective from its development [29].
Scientific findings on the role of interleukins present some contradictions, likely due to the complexity of cytokine interactions and disease pathways, as well as variability in experimental designs, methodologies, and study samples. Further investigations of the interplay between IL-27 and IL-30 may reveal novel pathways in the pathogenesis of psoriasis and potentially lead to developing new therapeutic targets.