We present the case of a 3-month-old boy with a large right pulmonary artery to left atrial fistula successfully treated by percutaneous closure. The 3D printing model was useful for planning and simulating the procedure and device selection.
The boy was born in good general condition with a weight of 3670 g and 10 points on the Apgar scale. No abnormalities were found in prenatal examinations. On the first day of life central cyanosis was found, with saturation of about 70%, and a heart murmur was heard. Based on the echocardiographic examination, the diagnosis of right pulmonary artery to left atrial fistula was made. To confirm the above diagnosis and exclude the coexistence of other vascular malformations, angio-computed tomography (angio-CT) with 3D reconstruction was performed.
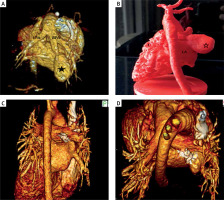
This examination confirmed abnormal division of the right pulmonary artery into 2 vessels: the proper pulmonary artery and the vessel leading to a 21 × 10 mm reservoir, which then in the place of the lower right pulmonary vein escaped through a wide opening (11 mm) into the left atrium. (Figure 1 A).
Figure 1
Computed tomography – reconstruction 3D: A – View of the arterial part of malformation. Visible trunk of the pulmonary artery with a left (LPA) and right (RPA) pulmonary arteries. The right pulmonary artery is divided into true RPA and a vessel supplying the malformation reservoir (*). B – 3D printed model of the patient’s heart in real scale. Visible venous drainage of the malformation’s reservoir (*) to left atrial (LA). C, D – CT – reconstruction 3D 20 months later – visible set in the correct position, confirming the good effect of the treatment
In the subsequent observation the child remained in a good general condition. Apart from slight desaturation (88–89% saturation) and a quiet murmur over the heart, he did not present any abnormalities. For this reason, the treatment decision was postponed to 3 months, then child’s body weight was 7.2 kg. The desaturation and the potential risk of neurological complication (high risk of paradoxical embolism) were the indications to perform percutaneous closure of the fistula.
To choose the most optimal access, type, and size of device, we decided to simulate a malformation closure using a printed 3D model. Based on the CT scan of the heart, we prepared 3D printed models: model No. 1 was a solid print representative of a full volume heart and vessels anatomy (Figure 1 B), and model No. 2 was a hollow print for simulation representing chambers and vessels in the region of interest. Using model No. 2, made of semi-flexible material visible under fluoroscopy, we performed a simulation of malformation closure from the pulmonary artery with the 6 mm Amplatzer Duct Occluder I device. The position of the device was stable and did not hinder the free insertion of the catheter into the right pulmonary artery (Figures 2 A–C).
Figure 2
The simulated procedure from the femoral vein. 3D model limited to the region of interest – right pulmonary artery (RPA) is divided into 2 vessels. Malformation reservoir is visible. A – The sheath introduced from femoral vein to right pulmonary artery and malformation reservoir. B – Amplatzer ADO is implanted from pulmonary artery site. C – The sheath introduced to true right pulmonary artery. The implanted set does not narrow the right pulmonary artery. D – Control patient’s heart scan – implanted Amplatzer Vascular Plug II
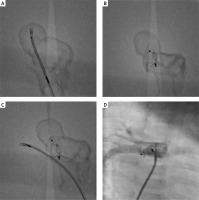
Finally, from the right femoral vein, a 6 × 6 mm Amplatzer Vascular Plug II was implanted [1–5]. The final choice of an Amplatzer Vascular Plug was based on our belief that this device is more likely to provide the best treatment outcome, i.e. to close the fistula without residual leakage and to maintain normal flow in the right pulmonary artery. In control angiography, we did not find any flow disturbances in the right pulmonary artery (Figure 2 D). The fluoroscopy time was 6.2 min, and the radiation dose was 28 mGy. During the procedure we used Heparin 100 IU/kg. Due to the large reservoir and the lack of progressive standards, we recommended acetylsalicylic acid 5 mg/kg/day for 6 months.
Twenty months after the procedure, a check-up CT was performed, confirming the good long-term effect of the treatment (Figures 1 C, D).
We are convinced that the use of 3D printing simulation significantly reduced the time of the procedure and increased its effectiveness and safety [6].








