Hepatic artery aneurysms (HAAs) are uncommon, accounting for approximately one-fifth of all visceral artery aneurysms [1]. Recognizing HAAs is crucial due to the potential for complications, such as rupture leading to gastrointestinal and intraperitoneal haemorrhage, and ultimately, patient mortality. The complete aetiology of these aneurysms is still under investigation, but it seems that risk factors include arteriosclerosis, portal hypertension, and, among others, systemic lupus erythematosus (SLE) [2].
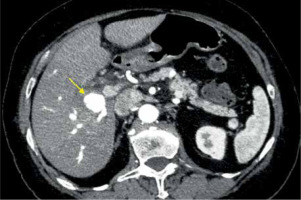
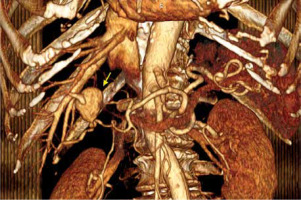
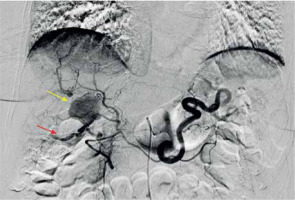
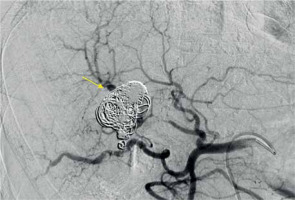
A 75-year-old Caucasian female with a history of arterial hypertension and untreated ascending aortic aneurysm presented at her local hospital during her first episode of acute cholecystitis. A computed tomography (CT) scan was performed at the hospital to investigate the cholecystitis, revealing an aneurysm in the anterior branch of the right hepatic artery, measuring 2.2 × 1.7 × 2.3 cm (Figures 1, 2). Initial treatment focused on managing the cholecystitis non-surgically and managing the aneurysm first, based on Tokyo guidelines [3]. Five days after admission, a follow-up CT scan indicated an enlargement of the aneurysm (2.3 × 2.0 × 2.9 cm), raising suspicion of a micro-rupture. Laboratory tests indicated elevated hepatic enzymes. Consequently, the patient was transferred to our clinic for further investigation. Angiography of the celiac branch revealed an intrahepatic aneurysm in a branch of the right hepatic artery (Figure 3), along with thrombosis in the posterior right portal vein, possibly due to external pressure from the aneurysm. No shunt between the aneurysm and portal circulation was evident. The aneurysm was then successfully embolized using metallic micro-coil springs. In addition, another branch of the right hepatic artery was found to be dysplastic in angiography; this raised a suspicion of vasculitis (Figure 4). The patient remained haemodynamically stable, and there was no further deterioration of liver function. Subsequently, total body CT angiography indicated no other aneurysms or other pathological findings and confirmed a successful embolization. The rest of the hospitalization was uneventful, and further testing identified SLE as the underlying cause. Cholecystectomy was scheduled after 6 weeks of discharge. Splanchnic artery aneurysms are rare, with a total prevalence of 0.5%, and they are significant due to the potential for rupture in up to 25% of cases [4]. Pseudoaneurysms are more common (with a prevalence of almost 50%) than true aneurysms, which is probably due to the increasing number of hepatobiliary procedures [5]. Clinical manifestations of HAA are typically absent and incidentally detected through imaging studies. When symptomatic, approximately one-third of patients present the classic Quincke triad of biliary colic, haemobilia, and obstructive jaundice [6]. Around one-quarter of cases manifest with intraperitoneal rupture or gastrointestinal haemorrhage, carrying an overall mortality rate of about 21% [7]. The exact cause of HAA remains unclear, but factors such as arteriosclerosis, fibromuscular dysplasia, portal hypertension, collagen vascular diseases, and autoimmune conditions like SLE, polyarteritis nodosa, and Takayasu arteritis probably contribute to its development [2, 4]. Computed tomography angiography (CTA) serves as the gold standard for diagnosing HAAs. While most patients with HAA are diagnosed incidentally through CT scans performed for other reasons, CTA is preferred due to its ability to localize and size the aneurysm, as well as detect other microaneurysms or vasculitis. Additionally, CTA allows for therapeutic intervention planning in cases requiring radiological or surgical treatment [7]. Treatment strategies depend on aneurysm location, size, and type. Commonly performed methods include open surgery (including ligation, lobectomy, and bypass surgery) and endovascular exclusion with coil embolization or flow diversion stent placement [8]. Treatment should be considered in all patients with symptoms related to the aneurysm if the aneurysm is more than 2 cm in diameter, if the patient is pregnant, or if there is demonstrated growth in the aneurysm [9]. For patients with incidentally discovered aneurysms, surgery has traditionally been an accepted management approach. A microcatheter is navigated into the aneurysm, packed with coils, and then the feeder vessel is embolized both distal and proximal to the aneurysm (front door-back door coil embolization). Ischaemia is not a usual complication due to the high collateral circulation that prevents ischaemic damage to the tissue supplied by the artery distal to ligation [5]. Although rare, it is important to recognize hepatic artery aneurysms due to the risk of rupture and the high mortality rate associated with these complications. HAAs can be safely treated with minimally invasive endovascular procedures such as stenting or embolization, while surgery remains a last resort in such cases.