Summary
We have previously reported that systemic-intraosseous delivery of dystrophin expressing chimeric (DEC) cells of normal (wt) and dystrophin-deficient (mdx) myoblast (MB) or mesenchymal stem cell (MSC) origin restored dystrophin expression and improved cardiac function in the mdx mouse model of Duchenne muscular dystrophy (DMD). Using the same mdx mouse model, this study confirmed that 0.5 × 106 of DEC therapy (MBwt/MBmdx and MBwt/MSCmdx), which combines the myogenic and/or immunomodulatory properties of MB and MSC, significantly improved the force and resistance to fatigue of skeletal (gastrocnemius) muscles at 90 days after systemic DEC administration as confirmed by standard functional tests.
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked neuromuscular disorder caused by a mutation in the dystrophin gene. Dystrophin deficiency results in myofibril damage, inflammation and fibrosis, and manifests as progressive skeletal and cardiac muscle weakness, which subsequently leads to cardiac dysfunction and failure [1–5]. Despite extensive research testing novel cell and gene-based therapies, no cure preventing or treating DMD exists.
We have introduced and tested, in vitro and in vivo, murine and human dystrophin expressing chimeric (DEC) cell lines created by ex vivo cell fusion of normal myoblasts (MB) and normal/dystrophin-deficient MB or mesenchymal stem cells (MSC) for future clinical application to treat DMD [1–3]. Both MB and MSC parent cells used for DEC creation presented desirable characteristics such as regenerative, anti-inflammatory and immunomodulatory potential for development of stem cell-based therapies targeting DMD. We have previously reported that after ex vivo polyethylene glycol (PEG)-mediated cell fusion, the chimeric state of murine and human DEC cells was confirmed by flow cytometry [1–4], confocal microscopy [1, 3, 4], polymerase chain reaction (PCR)-short tandem repeats and polymerase chain reaction-reverse sequence-specific oligonucleotide probe [1, 3, 4]. DEC expressed dystrophin and showed myogenic differentiation [2, 3] and decreased immunogenicity [3, 4]. Moreover, the safety of DEC was confirmed by COMET assay presenting no genotoxicity effect of the fusion procedure on the created DEC line [3, 4].
Our previous study confirmed that intraosseous delivery of murine DEC lines of normal MB (wild type – wt) fused with dystrophin-deficient MB (MBmdx) or (MSCmdx) significantly increased dystrophin expression in the cardiac muscle, which correlated with improved cardiac functions of ejection fraction and fractional shortening in MBwt/MBmdx and MBwt/MSCmdx DEC injected mice [3].
Aim
This study focused on evaluation of the systemic effect of intraosseous administration of MBwt/MBmdx and MBwt/MSCmdx DEC therapies on the function of the skeletal muscle assessed in gastrocnemius muscle (GM) of mdx mice using in situ and ex vivo muscle force testing.
Material and methods
Experimental animals
Animal care and experimental protocols were approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee (IACUC), accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC).
Six- to eight-week-old male mice – mdx (C57BL/10ScSn-Dmdmdx/J, stock number 001801) with the corresponding background snj wild type (wt) mice (C57BL/10ScSnJ, stock number 000476) were purchased from Jackson Laboratories. All animals received humane care with ad libitum access to water and normal chow in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animal Resources published by the US National Institutes of Health.
Ex vivo creation of dystrophin expressing chimeric (DEC) cells of myoblast and mesenchymal stem cell origin
The methodology of creating murine MBwt/MBmdx and MBwt/MSCmdx DEC has been reported previously [3, 4]. Briefly, DEC were created via ex vivo polyethylene glycol (PEG)-mediated fusion. The MBwt/MBmdx DEC line was created from myoblasts isolated from the mdx and snj wild type (wt) mice, whereas the MBwt/MSCmdx DEC line was created from the wild type (wt) myoblasts and MSC harvested from mdx mice as previously described [3].
Myoblast (MB) isolation
Minced muscles derived from donor mouse hind limbs were incubated with 1.5 U/l Collagenase type D (Roche–Thermo Fisher, Waltham, MA, USA) and 2.4 U/l Dispase II (Sigma, St. Louis, MO, USA) in 2.5 mM CaCl2 solution at 37°C for 30 min. Following digestion, muscles were further mechanically dissociated in primary culture medium (F-10+), containing Ham’s F-10 medium (Gibco-Thermo Fisher, Waltham, MA, USA), 20% Fetal Bovine Serum (FBS, Gemini Bio-Products, West Sacrament, CA, USA), 1% antibiotic-antimycotic solution (Gibco-Thermo Fisher, Waltham, MA, USA) and 2.5 ng/ml basic fibroblast growth factor (bFGF, PeproTech, Rocky Hill, NJ, USA). Cells filtered through 100 μm and 70 μm pore size nylon meshes (Thermo Fisher, Waltham, MA, USA) were counted and plated in 75 cm2 collagen-coated tissue flasks (Celltreat Scientific Products, Pepperell, MA, USA). Adherent cells, reaching 60–80% confluence, were harvested and pre-plated for 15 mina in a collagen-coated flask to eliminate fibroblasts. Non-adherent cells were transferred for further culture in F-10-based Primary Culture Media. After 3–4 additional passages and pre-plating steps, myoblasts (MB) were expanded in F10/DMEM-based Myoblast Growth Medium supplemented with 20% FBS (Gemini Bio-products, West Sacramento, CA, USA), 1% antibiotic-antimycotic solution (Gibco-Thermo Fisher, Waltham, MA, USA) and 2.5 ng/ml bFGF (PeproTech). MB at passages 4–6 were used for the fusion procedure [3].
Mesenchymal stem cell (MSC) isolation
Bone marrow cells harvested from the femurs and tibias of 6 weeks old mdx mice were washed and cultured in medium containing 40% alpha Modified Eagle Medium (αMEM), 40% F-12 nutrient mixture (Invitrogen, USA), 10% FBS (Gemini Bio Products, USA), and 1X antibiotic-antimycotic solution (Invitrogen, USA). After 7-day culturing, the adherent cells were depleted using MACS cell separation columns (Miltenyi Biotec, USA) with antibodies to CD11b and CD45 (eBiosciences, Imc. USA). The CD45-CD11b- subpopulation was cultured in 175 cm2 flasks (Nunc, USA) at a density of 1 × 106 cells per flask. MSC at passages 4–6 were used for the fusion procedure [3].
Ex vivo cell fusion procedure
The polyethylene glycol (PEG) fusion protocol was performed as previously described [3, 4]. Prior to fusion, parent myoblasts for MBwt/MBmdx DEC fusion and myoblasts and MSC for MBwt/MSCmdx DEC fusion were fluorescently labeled using either PKH26 or PKH67 (Sigma, St. Louis, MO, USA) tracking membrane dyes according to the manufacturer’s instructions. Fusion was performed using fusion medium consisting of 3.5 g of polyethylene glycol (PEG 4000, EMD), 400 μl of DMSO (Sigma) and 2 ml of serum-free DMEM medium supplemented with 1X Antibiotic/Antimycotic solution. Following washing in complete culture medium, the fused cells were transferred to fluorescently activated cell sorting (FACS) buffer containing 5% HEPES, 1% EDTA and 5% FBS. Cells presenting double (PKH26/PKH67) staining were selected by FACS (BD Astrios, BD Biosciences) and transplanted to the recipient mdx mice. The experimental study design is outlined in Figure 1. A total of n = 7 fusions were performed to create the two murine DEC lines MBwt/MBmdx and MBwt/MSCmdx and to assess DEC efficacy in vivo after systemic-intraosseous transplant to the mdx mice.
Figure 1
Diagram presenting creation of murine dystrophin expressing chimeric cell (DEC) using polyethylene glycol (PEG) mediated ex-vivo cell fusion procedure and systemic-intraosseous transplantation of murine DEC to the mdx mice. Ex vivo created DEC derived from myoblasts of wild type (MBwt) and dystrophin-deficient mdx mice MBmdx origin, or MBwt and mesenchymal stem cells of dystrophin-deficient mdx mice (MSCmdx). MBwt were labeled with PKH26 fluorescent dye and MBmdx or MSCmdx were labeled with PKH67 fluorescent dye. Following ex-vivo cell fusion DEC cells were sorted based on double positive PKH26/PKH67 labeling and were administered via the systemic-intraosseous route to the femoral bone of an mdx recipient mouse
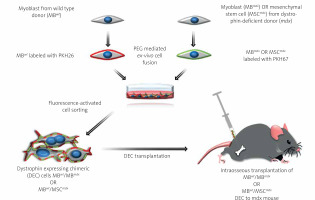
Murine DEC transplantation
Age-matched 6–8-week-old mdx recipients were randomly assigned to: Group 1 – vehicle injection (n = 6, 60 μl PBS), Group 2 – injection of non-fused MBwt and MSCmdx (n = 3, 0.5 × 106 in 60 μl DPBS), Group 3 – injection of fused MBwt/MSCmdx DEC (n = 3) and Group 4 – injection of MBwt/MBmdx DEC (n = 4, 0.5 × 106 in 60 μl DPBS). Cells were delivered to the right femur of an anesthetized mdx recipient mouse, as previously described [3, 5–7]. Briefly, following skin incision at the mid-femur level, subcutaneous tissue and the overlying muscles were dissected. Next, using a 28G needle 60 μl of bone marrow was aspirated from the recipient’s femur medullary cavity and DEC cells or controls suspended in 60 μl of sterile saline were administered to the created space using a tuberculin syringe with a 27G needle (Exelint International, Los Angeles, CA, USA). To prevent cell leakage, the injected bone was sealed with bone wax. The muscle and skin were closed using 4–0 monofilament absorbable suture. Animals recovered in a heated environment and were returned to the colony. The 90-day follow-up included observation of the DEC injection site, to assess the presence of ecchymosis, inflammation or infection.
Gastrocnemius muscle strength evaluation
The in vivo and ex vivo gastrocnemius muscle (GM) force measurements were performed at the 90-day endpoint.
In situ force test
In situ GM force measurements were performed under 1.5% isoflurane anesthesia. The Achilles tendon was dissected and tied with silk to a force transducer. The sciatic nerve was isolated and stimulated with a bipolar wire electrode. Muscle force was measured after optimal voltage and length were determined. Fatigue was measured after 10 min of submaximal tetanic stimulation as described previously [1, 2, 4]. The GM was kept moist during the whole procedure by continuous drip of Krebs-Henseleit solution (in mM: 130 NaCl, 5 KCl, 1 CaCl2, 1.1 KH2PO4, 0.85 MgSO4, 0.6 MgCl2, 25 HEPES, 25 NaCO3, 11 glucose bubbled with 95% oxygen and 5% carbon dioxide). The impact of the drip did not introduce mechanical artifacts. Optimal passive tension was determined by stimulating the sciatic nerve for 6 s. The passive tension was increased every 3 twitches until the maximum force was recorded. Optimal voltage was re-determined after each test. When the optimal voltage changed, the data from the previous test were discarded and the test was repeated. To ensure proper voltage, a set of twitches was elicited beginning at 1.0 V with a 1-ms pulse every 3 s with gradual increases in voltage until maximum force was obtained. The voltage used for the experiments was 1.2 times the optimal voltage determined and was usually 2.0 V. After optimal voltage and length were determined, the nerve was stimulated every 3 s with 1-ms pulses for 10 repetitions. The amplitude of the twitches and the rates of force generation and relaxation were measured. Twitches were repeated throughout the test to verify that the optimal voltage and passive tension were maintained. A 300-ms, 50-Hz burst of stimulation was applied to the nerve every 3 s for 10 min Fatigue is reported as the minimum force, usually at 10 min, as a fraction of the maximum tetanus force. Maximum tetanus typically occurred within the first several seconds of the fatigue experiment, and typically by the second recorded peak. In these measurements, a smaller number means greater fatigue. Potentiation was reported as the maximal force, usually within the first 40 s of the test, as a percent of the maximum tetanus force [1, 2, 4].
Ex vivo force test
The contractile and passive muscle forces of the GM were measured in vitro using the 1200A isolated muscle test system (Aurora Scientific, USA). Following mdx recipient euthanasia, the GM dissection including the Achilles tendon from right and left limbs was performed. GMs were placed in 37°C Krebs-Henseleit solution in a Radnoti glass chamber tissue bath. Silk ties were applied to attach the Achilles tendon and proximal pole of the muscles to the force transducer. The measurement of GM force was performed after establishing optimal length through a standardized stimuli pattern until reaching maximal wave and maximal strain [1, 2, 4].
Results
Improvement in gastrocnemius muscle (GM) functional outcomes was confirmed by standard in vivo functional tests assessed at 90 days after systemic-intraosseous DEC transplant to the mdx mice
Muscle force testing
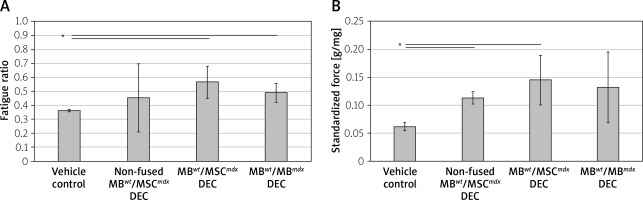
Muscle force testing showed significant improvement in the fatigue ratios in the mdx mice injected with MBwt/MSCmdx DEC (0.567 ±0.116, p < 0.05) as well as MBwt/MBmdx DEC cells (0.489 ±0.087, p = 0.015) compared to the vehicle controls. In contrast, there was no significant difference between the vehicle controls and mdx mice injected with non-fused cells, which showed a large variability in the response (Figure 2 A).
Figure 2
Systemic-intraosseous transplantation of MBwt/MBmdx and MBwt/MSCmdx DEC lines improved gastrocnemius muscle (GM) fatigue tolerance and force at 90 days after transplant to the mdx mice. In situ GM fatigue tolerance (A) GM force (B). Muscle force was normalized by the isolated GM weight. A smaller number in fatigue tolerance indicates more fatigue. *P < 0.05
Maximum force at tetanus
Maximum force at tetanus stimulation, which was standardized for gastrocnemius muscle weights, showed improvement in groups injected with non-fused MBwt and MSCmdx cells (0.113 ±0.040 g/mg of GM muscle, p = 0.022) and MBwt/MSCmdx cells (0.145 ±0.040 g/mg of GM muscle, p = 0.040) compared to the vehicle controls. Although the maximum force was not significantly increased in mdx mice injected with MBwt/MBmdx, there was a trend to an increase as well (Figure 2 B).
Maximum force generation rate and maximum force relaxation
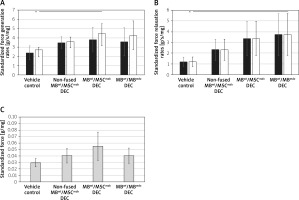
Maximum force generation rate was significantly increased in MBwt/MSCmdx fused DEC cells compared to vehicle-injected controls (4.45 ±1.1 vs. 2.7 ±0.3, p < 0.05, Figure 3 A), and the maximum force relaxation rate showed a significant increase in MBwt/MBmdx fused DEC cells compared to vehicle-injected controls (3.74 ±1.95 vs. 1.21 ±0.4, p < 0.05, Figure 3 B).
Figure 3
Comparison of in situ tested initial and maximum force generation and relaxation of gastrocnemius muscle (GM) as well as twitch amplitudes at 90 days after MBwt/MBmdx and MBwt/MSCmdx DEC lines after intraosseous administration to mdx mice. A – Initial and maximum force generation rates, B – Initial and maximum force relaxation rates (initial rates of force generation and relaxation are indicated by black bars; maximum rates of force generation and relaxation are indicated by white bars), C – single depolarization twitch amplitudes. Muscle force and twitch amplitudes were normalized by the isolated GM weight. *P < 0.05
Individual twitch amplitudes
Individual twitch amplitudes did not differ between treatment groups and non-fused and vehicle-injected controls (Figure 3 C).
Ex vivo functional outcomes assessed in gastrocnemius muscles (GM) at 90 days after systemic-intraosseous DEC transplant to the mdx mice
The ex vivo GM force testing in MBwt/MSCmdx DEC recipients detected an increase in the average GM force compared to the vehicle-injected and non-fused controls (32.3 ±3.2% vs. 25 ±9.2%, respectively); however, the value was not statistically significant (Figure 4 A). No differences were observed in the fatigue values among the assessed groups (Figures 4 B and C).
Discussion
Preventing the premature loss of mobility and early mortality of DMD patients through delivery and restoration of muscle dystrophin has been the aim of ongoing development for novel therapies for DMD such as exon skipping, gene editing via viral vectors, and stem cell transplants [8–11]. Although MB and bone marrow-based therapies have shown promise, the low engraftment rate caused by immune rejection and the necessity of supportive immunosuppression reduce the efficacy and feasibility of this approach in the clinic [10, 12–14]. However, they fueled the incessant search for new and more effective strategies of stem cell application in treating DMD [15–19]. Previous studies have demonstrated dystrophin expression and restoration of functional dystrophin following transplantation of the autologous cells engineered to express dystrophin via differentiation of pluripotent stem cells to muscle fibers to modify DMD pathology [16].
To address the limitations of current stem cell therapies, we have introduced dystrophin expressing chimeric (DEC) cells as a novel cell-based therapy for DMD. In previous studies, we have confirmed fusion feasibility and performed in vitro phenotype and genotype characterization [1, 2]. In vivo assessment of murine and human DEC confirmed DECs’ engraftment, long-term survival and improved skeletal and cardiac muscle function [1–4].
We have also confirmed the therapeutic effect of DEC after intramuscular DEC transplant to the gastrocnemius muscle (GM) of the mdx mice, which revealed over a 37.27% increase in dystrophin expression, almost two-fold higher than assessed by other laboratories as the level required to obtain functional improvement [2]. These results correlated with trends of improved muscle strength and function assessed by the wire hanging test and grip strength measurements up to 30 days after DEC transplant, despite the animal learning and behavioral factors [1, 2]. The current study continues and adds to our previously published data [2, 3] evaluating the effect of murine DEC cell lines of myoblast and MSC origin (MBwt/MBmdx and MBwt/MSCmdx, respectively) after systemic-intraosseous transplant to the mdx mice.
Our results suggest that both types of fused DEC lines studied significantly improved the fatigue ratio compared to vehicle-injected controls and non-fused cells, potentially serving as viable novel therapies for improving muscle performance in the DMD pathophysiology cycle. The standardized maximum tetanus force, representing the cumulative performance of all muscle units that comprise the GM during fatigue testing, increased for both treatment groups that received MSCmdx cells irrespective of fusion status. However, MSCmdx cells would not recover dystrophin production since they are derived from dystrophin-deficient mdx mice. Thus, most likely the MBwt cells were the ones responsible for the increase since they were present in all injections with MSCmdx cells.
Twitch amplitudes for all treatment groups, which represent the muscle’s ability to contract in response to a single depolarization event or stimulus, did not differ between study groups. Increases in maximum tetanus force without a corollary increase in twitch amplitude suggest that while muscle units on an individual level may not show clinically impactful improvements in function during a single depolarization, the concerted effort between all muscle units with repeat stimulation does demonstrate an amelioration in some of the deleterious effects of dystrophin deficiency. This may be explained by the fact that not all units restored dystrophin to the same extent. To what degree these interactions differ between DEC of myoblast origin (MBwt/MBmdx) when compared to DEC of MSC origin (MBwt/MSCmdx) has to be determined and will be a focal point for future studies.
One potential mechanism by which these two DEC lines may differ in their effects on myocytes is via ion channels and ATP pumps in the sarcolemma and sarcoplasm. The speed of membrane depolarization and efficiency of repolarization across the muscle unit is measured as the derivative of the voltage peaks generated by the force transducer, with the upward slopes of peaks representing force generation rates and downward slopes representing force relaxation rates [20]. Mice treated with MBwt/MBmdx had statistically significantly faster force relaxation rates, suggesting improved efficiency in repolarization compared to other treatment groups. Since mdx myoblasts have already undergone differentiation compared to mdx mesenchymal stem cells, they may have more preexisting cellular infrastructure in their cell membranes such as ion channels, ATP pumps, carrier proteins, etc. Therefore, fusing wild type and mdx myoblasts may result in more ion channels in their resultant shared cell membrane and other proteins involved in relaxation during the excitation-contraction coupling mechanism, leading to a more efficient repolarization response and higher force relaxation rates [20].
Intraosseous injection of both DEC cell lines showed significant improvement in muscle function when compared to vehicle-injected controls and injection of non-fused cells, thus confirming the systemic effect of DEC therapy. These findings are in line with our previous studies confirming better engraftment after intraosseous compared to intravenous transplantation of cell-based therapies [5–7]. Moreover, we reported that the intraosseous delivery route of DEC resulted in protection of cardiac function in the mdx mouse model as confirmed by echocardiography, which revealed the rebound effect on the ejection fraction and fractional shortening observed at 90 days after systemic-intraosseous DEC administration when compared to the vehicle-injected controls [3].
These studies support the intraosseous delivery route as a preferable method for intramuscular delivery of DEC cells, which generate only a local and not a systemic effect due to the limited migration of the locally injected cells. In addition, intraosseous delivery is a short, straightforward procedure which lasts several minutes, whereas intramuscular delivery is a tedious, lengthy procedure under anesthesia, which is less optimal in the clinical scenario when delivered to the pediatric population of DMD patients. Our previous study, evaluating the effect of MBwt/MBmdx and MBwt/MSCmdx DEC therapy on cardiac muscle, confirmed the protective systemic effect of DEC therapy administered via intraosseous injection, and is in line with the results of the current study [4].
It is important to emphasize that functional improvements in gastrocnemius muscle were assessed at 90 days after DEC transplant in the immunocompetent mouse model without immunosuppressive therapy. This further confirms the immunomodulatory effects of DEC cells [4].
In summary, in this study we confirmed the systemic effect of DEC cells of both MB and MSC origin leading to improved function in the skeletal muscle (GM) after intraosseous transplant to the mdx mouse.
Conclusions
DEC therapy represents a novel concept of ex-vivo fusion of normal and DMD affected myoblasts and MSC to enhance engraftment, elicit immunomodulatory effects, and thus minimize or eliminate the need for immunosuppression. The ultimate goal is to bring DEC therapy closer to clinical application in DMD patients, after verifying the safety and efficacy of DEC treatments with regards to functional outcomes.









