Introduction
Liver cirrhosis is the end stage of chronic liver diseases. Progression of liver fibrosis leads to an increase in the intrahepatic resistance to blood flow from the portal vein. Portal hypertension (PH) can be defined as an increase in hepatic venous pressure gradient (HVPG), which is a surrogate of blood pressure in the portal vein, to values ≥ 5 mmHg. Clinically significant PH is defined as HVPG > 10 mmHg. The increase of pressure in the portal vein and its branches leads to compensatory opening of the shunts between the portal vein and systemic venous circulation. The most clinically important shunts are esophageal varices in which short-term prognosis of endoscopic treatment was predicted by HVPG. Furthermore, HVPG is considered a marker of liver cirrhosis decompensation [1, 2], risk of development of hepatocellular carcinoma (HCC), and correlates with overall prognosis of patients with liver cirrhosis [3, 4]. HVPG can be measured by hepatic vein catheterization. The indications for the procedure may be an estimation of the risk of rebleeding after endoscopic treatment of acute variceal hemorrhage, transjugular liver biopsy in patients with coagulation disorder, evaluation of non-selective β-blocker therapy efficacy, and risk stratification of postoperative outcomes in patients with liver cirrhosis and HCC. The last indication applies to patients with potentially curable HCC. Guidelines of the European Association for the Study of the Liver (EASL)/European Organization for Research and Treatment of Cancer (EORTC) from 2012 defined criteria for candidates for liver resection as follows: 1) The patient should have a single lesion, 2) well-preserved liver function, 3) HVPG ≤ 10 mmHg or 4) platelet count ≥ 100,000/ml [5]. These criteria decreased the risk of severe decompensation and perioperative lethality; however, they are considered quite restrictive. Recently there is a visible shift to go beyond the aforementioned criteria at tertiary centers. It is necessary to consider the presence of clinically significant portal hypertension (CSPH) and particularly use the most minimally invasive surgical technique that can be curative [6, 7]. The latest EASL HCC guidelines from 2018 [8] reflect this new trend in curative therapy for HCC. A patient who is not a candidate for liver transplantation (Tx) should be considered for liver resection with thorough risk assessment. The resection is potentially curable, but on the other hand, it can lead to fatal liver decompensation. When surgery is contraindicated, the other treatment modalities are always only palliative, with significantly worse outcomes [9]. In the following text, the authors present their own experience with HVPG measurement before surgery in cirrhotic patients with HCC and provide the procedure description.
Material and methods
We retrospectively evaluated patients with HCC who were candidates for liver resection according to standard criteria including HVPG. We described the frequency of contraindications to resection based on HVPG, overall survival (OS), perioperative morbidity, and lethality from liver causes on the 30th and 90th day after resection, and the rate of complications of hepatic vein catheterization. The MedCalc program version 20.106 (MedCalc Software Ltd., Ostend, Belgium) was used for statistical analysis. The survival analysis was performed by the Kaplan-Meier method and comparison between groups was assessed by the log-rank test. The Institutional Review Board was consulted, and patient consent was waived due to the study’s retrospective and observational character.
Own experience
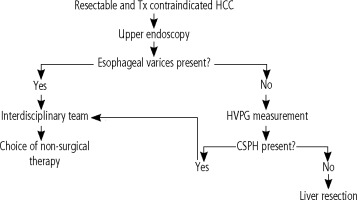
The interdisciplinary group at the Military University Hospital in Prague (MUH) is involved in complex treatment management of patients with HCC. Within the workplace, an algorithm was adopted for the examination of a patient with early HCC based on the Barcelona Clinic Liver Cancer classification (BCLC) who was contraindicated and/or refused a transplant (Fig. 1). The treatment management is based on the consensus of an interdisciplinary team, consisting of a hepatologist, a hepatobiliary surgeon, a radiologist experienced in focal liver lesions, and an oncologist. In the case of resectable HCC according to CT imaging, an upper endoscopy is indicated, regardless of the number of platelets, to rule out esophageal varices and portal hypertensive gastropathy as manifestations of PH. In the absence of PH signs and a potentially resectable tumor, all candidates undergo HVPG measurement with regard to the routine availability of the procedure at the Department of Medicine of the 1st Faculty of Medicine of the Charles University Prague and MUH. According to our algorithm the patient is a candidate for resection if significant CSPH is ruled out.
Description of hepatic vein catheterization and HVPG measurement
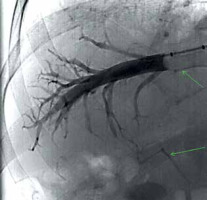
The examination begins with a patient interview consisting of an explanation of the procedure, and the signing of informed consent to the procedure and the iodine contrast solution administration (Iomeron). During the procedure the patient is placed on the fluoroscopy table in a supine position, peripheral venous access is always ensured, and vital functions are continuously monitored, including the ECG curve. After disinfection of the skin on the neck and subclavian area with alcohol and iodine solution, the entire area is sterilely draped. Applying the aseptic approach during the whole procedure the puncture site is anesthetized by local anesthesia and a needle is inserted into the right internal jugular vein following the welldescribed Seldinger method. In principle, the procedure up to this point does not differ from the insertion of a central venous catheter. To ensure the most direct course of the introduced instrumentation into the inferior vena cava for transjugular liver biopsy, a supraclavicular approach is preferred to more cranial one (Fig. 2). The next procedure is modified according to the current anatomical conditions, whereby either a direct catheter or a balloon occlusion catheter can be used. The advantage of the balloon catheter is that the measurement of hemodynamics covers a larger area of the liver parenchyma; however, the accuracy of the direct catheter measurement can be increased by multiple measurements at several locations, i.e., in several branches of the middle or right hepatic vein. The entire performance takes place under fluoroscopic control. The chosen type of catheter is the cannulated right or middle hepatic vein. Verification of the catheter position is done by administering a small dose of contrast solution. In the case of a satisfactory position, when the catheter floats in the vessel about 2 to 4 cm from the exit of the hepatic veins into the inferior vena cava and the contrast fluid flows around it completely free, actual measurement can be done. An infusion line and a pressure transducer with the possibility of continuous pressure curve recording are connected to the catheter. Free hepatic vein pressure (FHVP) is measured at this time. It is necessary to ensure sufficient time for the curve to stabilize. If a direct catheter is used, for the next measurements, it needs to be introduced as peripherally as possible so that it wedges in the periphery of the vein. At this moment, the wedged hepatic vein pressure (WHVP) is measured. To produce reproducible data it is important to wait for the hemodynamic conditions to stabilize (approx. 60 s). This procedure is repeated at least three times and the individual measurement values of both pressures are averaged. The accuracy of the overall measurement can be increased by wedging the straight catheter in different branches of the hepatic vein. The balloon catheter overcomes the necessity of peripheral insertion by vein wedging at the place of FHVP by inflating the balloon. Adequate occlusion is verified by administration of a small amount of contrast medium, which should stagnate in the vein (Fig. 3). With this step, we also rule out possible intrahepatic shunts between the portal and systemic veins in the measurement area. The difference between the FHVP and WHVP determines the gradient between the portal and systemic venous pressure – HVPG. Uncomplicated hepatic vein catheterization with HVPG measurement takes approximately 15-20 minutes, with an average contrast medium consumption up to 40 ml.
Cohort
In the period 1/2016-1/2023, preoperative HVPG measurements were performed in a total of 35 patients (30 males) with a mean age at the time of HCC diagnosis of 69.5 years (SD = 7.8). The basic demographic data of the patients are summarized in Table 1.
Table 1
Basic demographic data of the patients
Results
Hepatic vein catheterization was successful in 32 patients (91.4%). In 3 cases (8.6%) HVPG could not be measured due to the technical failure caused by a newly formed thrombosis of the middle and right hepatic veins in one case and anatomical abnormalities caused by severe scoliosis with a high diaphragm state preventing the hepatic veins being reached in the second case, malinsertion of the introducer into the right pleural cavity with the development of a pneumothorax in the third case. The aforementioned pneumothorax during central vein puncture or sheath insertion was the only complication of the procedure in the presented cohort (2.9%). Patients in whom it was not possible to determine HVPG were managed individually. Patient 1 with hepatic vein thrombosis was biopsied during the same procedure and advanced fibrosis was ruled out histologically and the patient underwent resection after a consensus was reached. Patient 2 with severe scoliosis underwent transarterial hepatic chemoembolization (TACE). Patient 3 was managed with chest drainage and after the stabilization the interdisciplinary team suggested a diagnostic laparoscopy due to low suspicion of CSPH. Intraoperatively the diagnostic laparoscopy was converted to liver resection due to favorable macroscopic liver findings lacking the signs of cirrhosis.
All 35 patients were initially candidates for liver resection. Tx was not indicated before hepatic vein catheterization for the following reasons: in 5 cases (14.3%) due to persistent alcohol abuse, 7 patients (20%) were over 75 years old at the time of liver tumor diagnosis, 2 patients refused the Tx (5, 8%), 1 patient was disqualified from Tx due to previous HCC rupture (2.9%), 5 patients had a positive history of oncological disease that was not in remission (14.3%). Some patients fulfilled multiple Tx exclusion criteria. A total of 17 patients (48.7%) were not Tx candidates before the HVPG measurement. Twenty patients (57.2%) eventually underwent liver resection. Based on 32 successfully performed HVPG measurements, resection for CSPH was contraindicated in 10 patients (31.3%), of whom 5 were selected for Tx (15.6%), 3 underwent TACE (9.4%), 1 refused treatment (3.1%), and in 1 case there was rapid disease progression with only systemic treatment (3.1%).
A total of 17 patients with preoperative favorable HVPG (≤ 10 mmHg) underwent elective resection. In one case, severe decompensation of liver cirrhosis with torpid ascites, hyperbilirubinemia, and coagulopathy occurred immediately after surgery (5.9%). One patient developed postoperatively infected ascites secondary to fasciitis (5.9%) None of the remaining 15 patients were readmitted for surgical complications and/or manifestations of liver insufficiency by day 90 after surgery. None of the resected patients died within the 30th or 90th day after surgery. One patient has not yet reached the 90th postsurgical day.
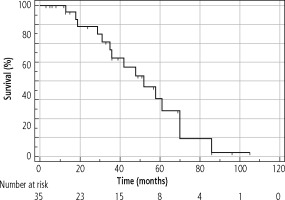
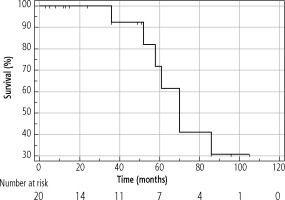
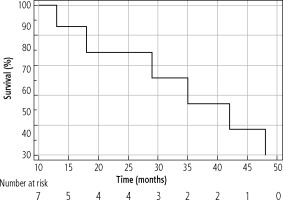
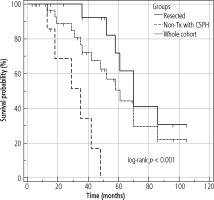
The median OS of the entire cohort was determined using the Kaplan-Meier method; it was 61 months (95% CI: 42-86) and is presented in Figure 4. The median OS in resected patients was 70 months (95 % CI: 52-86); see Figure 5. Patients who were contraindicated for resection based on evidence of CSPH and at the same time did not undergo Tx (for example, for progression on the waiting list for Tx) were treated with palliative methods and achieved a median OS of 35 months (95% CI: 13-48), as shown in Figure 6. The difference between groups was statistically significant with p < 0.001 and is presented in Figure 7. In the studied, highly selected cohort, 3 individuals finally underwent Tx and had not yet reached median OS.
Discussion
The rate of successful HVPG measurements in the presented cohort was more than 91%. In addition, in one patient, the failure was not due to technical reasons, but to the progression of the disease with thrombosis formation in the hepatic veins. Catheterization of the hepatic veins was diagnostic in this case. Our success rate is comparable with the 95% technical success of catheterization reported by Bosch [10].
The relatively high percentage of complications in the our cohort can be explained by the small sample size. As pneumothorax is a complication of central vein puncture, a comparison with this method is appropriate. Cannulation of the central vein is a significantly more common procedure than catheterization of the hepatic veins, and according to the literature, the risk of pneumothorax ranges from 1% to 6.6% [11, 12]. Naturally, hepatic vein catheterization, as an elective procedure, must be subjected to maximum demands to reduce the risk of complications including pneumothorax.
In 17 cases (48.7%), the possibility of Tx was ruled out even before the HVPG measurement. Therefore in this subgroup, resection was for these patients the only possible curative approach. In the case of flat-rate refusal of resection, many patients would be referred for palliative treatment, with significantly worse overall survival. The exclusion of CSPH thus enabled almost half of the patients to undergo a resection procedure with an acceptable level of perioperative risk. Hepatic vein catheterization with measurement of HVPG, possibly in combination with transjugular liver biopsy, may be the only diagnostic method to prove the presence of portal hypertension, and even the liver cirrhosis itself for some patients. These situations mainly include large tumors located in the right lobe of the liver. According to the Baveno VII Consensus, it is possible to use non-invasive liver stiffness measurement (LSM) methods for quantification of portal hypertension in patients with chronic advanced liver disease. However, there is a wide grey zone between the values 15 and 25 kPa, where CSPH cannot be ruled out. For routine praxis LSM is appropriate; on the other hand, if we evaluate a patient for high-risk curative surgery, HVPG can be the crucial examination to support our decision [13]. We compared data from both LSM and HVPG in 22 patients. Theoretically elasticity of 25 kPa should be diagnostic of CSPH [13]. However, in our cohort values of liver elasticity more than 25 kPa failed in 2 patients in whom CSPH was excluded by HVPG measurement. They represented 25% of patients with elasticity more than 25 kPa. The reason for misdiagnosis was in both cases the large tumor in the right lobe. The size of the lesion disqualified the patients from Tx and its location did not allow reliable LSM. In this case, hepatic vein catheterization plays a key role in patient management. We would like to point out that an essential condition for favorable results in terms of low perioperative morbidity and, in our cohort, zero mortality, is the extensive experience of the surgeons with liver resection in patients suffering from chronic liver disease.
Conclusions
Hepatic venous pressure gradient measurement is the gold standard for the quantification of PH. In the hands of an experienced examiner, this is a safe procedure that plays a fundamental role in the correct indication and postoperative risk assessment of surgical treatment of patients with primary malignant liver tumors and thus directly influencing their prognosis.