INTRODUCTION
Hereditary angioedema (HAE) is a rare autosomal dominant disease characterised by recurrent and unpredictable mucocutaneous oedema, with an estimated prevalence of 1 in 50,000 individuals [1]. Type I and II HAE are caused by mutations in the SERPING1 gene, which encodes the C1 esterase inhibitor protein (C1INH). High-molecular-weight kininogen is broken down to form bradykinin, which binds to bradykinin receptors and causes increased vascular permeability, vasodilation, and plasma leakage into the extracellular space, leading to angioedema [2]. Patients typically present with cutaneous angioedema without urticaria, abdominal symptoms resulting from gastrointestinal mucosal angioedema and respiratory symptoms caused by oropharyngeal/laryngeal angioedema [3]. Angioedema exacerbations are nonpruritic and do not respond to corticosteroids and antihistamines [4]. The diagnosis is established on the basis of serum levels of C4 and C1INH and the C1 inhibitor function. Serum C4 level is used for disease screening. If the C4 level is low, the C1INH level and activity are evaluated. Low C1INH level or function is suggestive of HAE due to C1INH deficiency (HAE-C1INH). In type I HAE, the C1INH level and function are inadequate, whereas in type II HAE, the C1INH function is inadequate despite normal or increased C1INH level [5]. Approximately 80–85% of patients have type I HAE and 15–20% have type II HAE [6]. Considering that 75% of patients have a family history of angioedema exacerbations, family members should also be screened. Both males and females are equally affected. The diagnosis is established based on the clinical symptoms, positive family history, and complement levels [7].
Patients with HAE experience a variable clinical course due to differences in symptoms. If left untreated, angioedema can progress rapidly within a few hours and typically lasts for 2–5 days [8]. Angioedema involving the neck may extend to involve the upper respiratory tract. Laryngeal involvement, the most severe complication, may be life-threatening by causing asphyxia. Abdominal colicky pain due to intestinal wall angioedema can be severe and mimic symptoms of acute abdomen, sometimes leading to unnecessary laparotomy [9]. Extremity exacerbations affect 96% of the patients. These exacerbations limit activity of the patients. Like extremity exacerbations, urogenital exacerbations are irritating, although they are not life-threatening [10]. Prompt treatment administered at the onset of an exacerbation significantly reduces morbidity and mortality. While treatment should be considered for all exacerbations, those affecting the abdomen and upper respiratory tract require immediate treatment. Treatment should be individualised based on the needs of each patient because the disease course can vary widely [11]. The disease severity, as determined by the patient’s subjective symptoms, should be supported by objective laboratory markers. The frequency and severity of exacerbations may be predicted by laboratory parameters that vary among patients [12].
AIM
In our study, we aimed to clarify whether there is an association of patient’s annual number of exacerbations with C1INH level and function. We, thus, tried to determine the predictive value of laboratory parameters used for diagnosis of HAE for varying numbers of exacerbations.
MATERIAL AND METHODS
STUDY DESIGN
The study protocol was approved by Necmettin Erbakan University Committee of Ethics in accordance with principles of Helsinki Declaration (1975) (approval no. 202/4218). For the study, a total of 53 patients who were being followed-up between January 2018 and December 2023 in Clinical Immunology and Allergy Clinic of Necmettin Erbakan University Faculty of Medicine were recruited. We investigated the associations between complement levels at the time of diagnosis and the annual number of exacerbations and disease severity. Demographic data, clinical histories, and laboratory results were obtained from patient files and electronic medical records. The inclusion criteria for the article were individuals aged 18–65 with a diagnosis of HAE. Patients who did not maintain regular outpatient follow-up or had lost contact were excluded from the study. Patients with an annual number of exacerbations > 8, regardless of the type of exacerbation, were categorised as severe cases, whereas those with 0–8 exacerbations annually were categorised as mild [13]. Additionally, information regarding the age at which patients experienced their first angioedema exacerbation and the sites of these exacerbations was collected. All participants provided written informed consent prior to their inclusion in the study.
BLOOD SAMPLING
Blood samples were centrifuged at 4000 rpm for 5 min at room temperature. They were kept at –20°C until the level of C1INH function was tested.
COMPLEMENT SYSTEM
The C1INH level was measured using a Neph 630 device (Siemens, Erlangen, Germany) based on the nephelometric method. The C1INH function was evaluated using a coagulation device (Cs-2500; Siemens) based on the chromogenic method. The C4 level was measured using an Architect C8000 device (Abbott, Des Plaines, IL, USA).
STATISTICAL ANALYSIS
Continuous variables are presented as medians with interquartile range (IQR) and were analysed using the Mann-Whitney U test, whereas categorical variables are presented as numbers with percentages and were analysed using the χ2 test. Spearman’s correlation test was used to evaluate the associations between the number of exacerbations and C1INH level and function and age at disease onset. SPSS software (version 22.0; IBM Corp., Armonk, NY, USA) was used for statistical analyses. P-values < 0.05 were considered indicative of statistical significance.
RESULTS
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS
The study included 53 patients with HAE due to C1INH deficiency, of whom 33 (62.3%) were females. No significant differences were found between sexes in terms of the HAE type, exacerbation frequency, or C1INH level or activity at the time of diagnosis. The median age of the patients was 41 (31–50) years, and the median age at symptom onset was 13 (7–18) years. Type I and II HAE were present in 33 (62.3%) and 20 (37.7%) patients, respectively. Mucocutaneous angioedema was the most common manifestation (96.2%), followed by gastrointestinal (69.8%) and laryngeal (54.7%) angioedema. At least one laryngeal involvement was observed in 17 (51.5%) and 12 (60%) of patients with type I and II HAE, respectively. No significant differences were observed in the number of exacerbations before and after treatment in patients with type I or type II HAE. The median number of annual pretreatment exacerbations was 24 (12–48) (Table 1).
TABLE 1
Demographic, clinical, and laboratory parameters of HAE-C1INH patients
ASSOCIATION BETWEEN COMPLEMENT LEVELS AND DISEASE SEVERITY
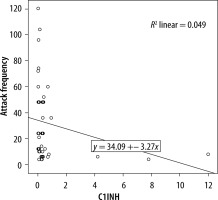
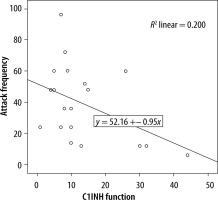
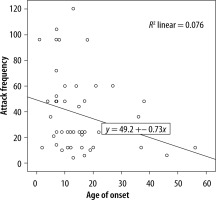
We evaluated the associations between complement levels at the time of diagnosis and the number of exacerbations experienced by patients. A negative correlation was observed between the number of exacerbations experienced by patients with type I HAE and C1INH level (r = −0.165, p = 0.002) (Figure 1). Furthermore, a negative correlation was observed between the C1INH function and the number of exacerbations in patients with type II HAE (r = −0.448, p = 0.038) (Figure 2). No significant associations were observed between the age at symptom onset and C1INH level or function. However, the age at symptom onset was negatively correlated with the number of exacerbations (r = −0.311, p = 0.028) (Figure 3).
COMPARISON OF PARAMETERS BY EXACERBATION FREQUENCY
Frequent exacerbations, defined as > 8 exacerbations per year, occurred in 21 patients. The C4 level and C1INH level and function were lower in patients with frequent exacerbations than in those with infrequent exacerbations (p = 0.018, 0.021, and 0.012, respectively) (Table 2).
TABLE 2
Comparison of demographic and laboratory parameters of HAE patients according to exacerbation frequency
DISCUSSION
We found a negative correlation between the C1INH levels at the time of diagnosis and the annual number of angioedema exacerbations in patients with type I HAE. Furthermore, in patients with type II HAE, there were significant negative correlations between the C1INH function at the time of diagnosis and the number of exacerbations. In addition, there was a negative correlation between age of onset of symptoms and number of exacerbations in HAE patients.
HAE involves the dysregulation of various physiological pathways, including the plasma kallikrein system, complement cascade, and coagulation system. Inadequate production or dysfunction of C1INH leads to an increased bradykinin level, which plays a central role in the pathogenesis of the disease. Angioedema affecting submucosal tissues can be particularly intolerable during episodic episodes. Understanding the course of these oedemas and determining appropriate treatment doses are crucial to managing the disease. Studies on predicting the course of these oedemas and adjusting treatment doses have taken into account the frequency of exacerbations [4]. Assessing disease severity is essential before initiating treatment because clinical manifestations of the disease can vary widely among patients. Subjective symptoms of the patients are used for rating of disease severity. Nonetheless, patient-dependent ratings such as annual number of exacerbations remain incapable. Early diagnosis of unpredictable angioedema episodes is crucial for managing potentially life-threatening exacerbations. Moreover, objective evaluation of disease severity is warranted to make a patient suitable for treatment. This evaluation may facilitate monitoring of disease activity and selection of appropriate patients for a given treatment. Therefore, biomarkers involved in the pathophysiology of HAE are being investigated as potential diagnostic and monitoring tools, which may be used for the diagnosis and follow-up of patients [14].
Measurements of C1INH level and level of C1INH function, which are used to diagnose HAE-C1INH, have also been investigated for use in determining disease severity. Levels of these parameters used for diagnosis of HAE may alter following treatment with C1 inhibitor concentrates or danazol [14]. Our study thus relied on clinical and laboratory data obtained at the time of diagnosis of HAE patients.
In a previous study investigating factors changing disease progression, a positive correlation between C1INH concentration and C1INH function was revealed. There was a negative correlation of both C1INH level and functional percentages with symptom severity score. C1INH concentration was negatively correlated in those with frequent symptoms. It was emphasised that the disease symptom score was higher in those with a C1INH activity of less than 10%, compared with those with a C1INH activity of more than 10% [15]. Another study compared the complement levels among HAE patients with varying disease severity, revealing a significant association between the C1INH activity and disease severity. Conversely, no association of CH50, C4, and C1INH levels with disease severity determined by the number of exacerbations was found. Nevertheless, age, disease severity score, and complement parameters did not differ between female and male patients [12]. In a similar study, the annual number of exacerbations and the number of C1 inhibitor concentrates used for treatment were compared with complement parameters. Because patients requiring emergency treatment for severe exacerbations experienced greater number of exacerbations, a significant correlation was determined. Lower C1INH and C4 levels were found to be associated with both increased number of exacerbations and number of C1INHs used for treatment. Furthermore, the negative correlation between C4 level and number of exacerbations was strong, and this study has shown that the level of C1INH function might be a predictor of disease severity [13]. Another study reported that a low C1INH function level was associated with increased annual number of exacerbations, and it had a negative impact on disease course by enhancing disease severity [16]. In our study, we found a significant negative correlation between the annual number of exacerbations and the C1INH level in patients with type I HAE. Also, we found a significant negative correlation between the C1INH function level and number of exacerbations in patients with type II HAE.
Previous studies have demonstrated that the age at symptom onset is a significant predictor of disease severity in HAE-C1INH. In a previous study, patients with a younger age of symptom onset were shown to have more frequent exacerbations [17]. A recent study demonstrated that earlier symptom onset is associated with a more severe disease course [18]. However, another study found that early symptom onset was associated with diagnostic delays and high annual number of exacerbations and number of hospitalisations. Therefore, earlier disease onset is associated with more severe disease, leading to a negative impact on the lives of patients [19]. It has been revealed that younger age of onset and lower levels of C1INH function might negatively influence the disease course [16]. In our study, patients with 0–8 exacerbations annually were categorised as having mild disease, whereas those with > 8 exacerbations per year were categorised as having severe disease [13]. Similarly to previous studies, we found that patients with a younger age at symptom onset had a higher annual number of exacerbations. However, we could not determine any significant association between age at symptom onset and C1INH level and function.
The main limitations of the present study were its retrospective design and small sample size. However, these factors were unavoidable because of the rarity of HAE.
CONCLUSIONS
We found a negative correlation between the basal C1INH reserve level and activity at the time of diagnosis of HAE and the number of exacerbations. In addition, we also found a negative correlation between age of onset of HAE symptoms and number of exacerbations. Exacerbations may not occur in some HAE patients, whereas they may be quite frequent in others. The frequency of exacerbations, which is different between patients, may change over time in the same patient. Therefore, it becomes difficult to determine the appropriate treatment for patients. Therefore, defining novel parameters in addition to using clinical evaluation for predicting disease severity would facilitate monitoring of the disease course [20, 21]. Our results demonstrate that the C1INH reserve level and function at the time of diagnosis can predict the disease severity in patients with HAE.