Introduction
Post-hepatectomy liver failure (PHLF) is a serious complication following liver resection (LR) and is an important cause of post-operative morbidity and mortality. Its incidence is variable and has been reported to range from 0.7% to 35% [1]. To reduce this risk, guidelines state a minimum of future liver remnant (FLR) > 20-25% in healthy patients without underlying liver disease prior to extended hepatectomy [2]. Associating Liver Partition and Portal vein ligation for Staged hepatectomy (ALPPS) is a two-staged procedure aimed to induce rapid hypertrophy of the FLR to reduce risk of PHLF, with higher successful completion of second stage of hepatectomy (98% vs. 78%, odds ratio [OR] = 5.75, p < 0.001), R0 resection (66% vs. 37%, OR = 4.68, p < 0.001) and shorter waiting time between the first and second stage of hepatectomy (11.6 vs. 45.7 days, weighted mean difference [MD] = −35.3 days, p < 0.001) compared to conventional two-stage hepatectomy (TSH) [3].
ALPPS was first reported by Schnitzbauer et al. in 2012 [4]. Their study reported a median volume increase of 74% in remnant liver volume (RLV) at a median of 9 days following stage 1 ALPPS, but with a high perioperative mortality of 12% [4]. While initial studies show high perioperative mortality, a recent metaanalysis comparing ALPPS vs. conventional TSH in 409 patients showed comparable peri-operative mortality, though with higher incidence of overall minor complications (59% vs. 18%, OR = 6.5, p < 0.001) [3].
Several methods to assess the interstage liver function and volume have been established [5]. While the use of hepatobiliary scintigraphy (HBS) was traditionally used to assess liver function due to its ability to selectively evaluate regions of the liver, studies have shown a poor correlation between functional increase versus volumetric increase, where FLR has been overestimated by up to 50% [6, 7]. Indocyanine green retention at 15 min (ICG-R15) is an alternative method which assesses global liver function based on the proportion of ICG remaining after 15 minutes. Reports have been equivocal on the trend of ICG-R15 pre-operatively compared to after stage 1; in one study ICG-R15 decreased from 9.9% to 7% between stages but increased to 33% after stage 2 [8], while in another study there was an increase in ICG-R15 from 3.6% to 5.4% postoperatively [9]. Volumetric assessment is performed through computed tomography (CT) to correlate with the extent of cellular proliferation [10].
While ALPPS results in a rapid increase in FLR, HBS studies have suggested that CT volumetry overestimates liver function increase [6]. In view of the lack of research investigating the correlation between ICG-R15 and CT volumetry results following stage 1 ALPPS, the primary aim of this study is to compare the extent of FLR increase compared with ICG-R15 before and after stage 1 ALPPS, as well as to correlate ICG-R15 and CT volumetry with incidence of PHLF. Our secondary aim is to review short-term and long-term outcomes of patients who underwent ALPPS in our tertiary centre.
Material and methods
This is a single-centre retrospective case series of 10 patients who underwent ALPPS between inception (May 2015) and January 2022 at our university-affiliated tertiary hospital. The exclusion criterion was being unable to complete the second stage of ALPPS (n = 1). This study was conducted in accordance with the PROCESS guidelines for reporting of surgical case series [11]. De-identified data were pre-collected using a prospectively maintained institutional liver database (Ref: TTSH/2018-00048). No attempts were made by the study team to access patients’ electronic medical records, or contact the included patients. This study was approved by our institutional review board (Ref: 2021/00898).
Study variables and outcomes
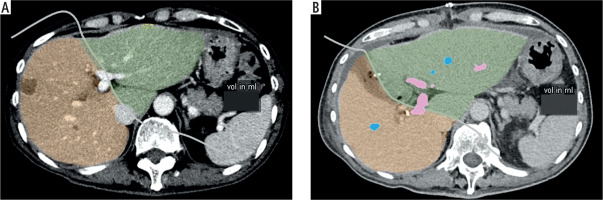
Study variables include age, gender, comorbidities, clinical presentation, pre-operative investigations (biochemical investigations and ICG-R15), CT volumetry (residual liver volume (RLV), standardized liver volume (SLV), and standardized future liver remnant (sFLR), histopathology findings and intra-operative findings (estimated blood loss, length of operation). SLV (ml) was calculated using the Chengdu formula: 11.508 × body weight (kg) + 334.024 [12]. sFLR is defined as the ratio of RLV to SLV. Figure 1A and B shows the method of calculation of the RLV of one of the included patients before stage 1 ALPPS and after stage 1 ALPPS respectively. Short-term study outcomes include the length of hospitalization stay (LOS), post-operative morbidity, 30-day readmission and 30-day mortality. Post-operative morbidity was defined as occurrence of any of the following post-operative complications: PHLF, intra-abdominal collection, pneumonia, pleural effusion, surgical site infection and ileus. 30-day readmission and 30-day mortality were defined as readmission or mortality, respectively, within 30 days from the date of stage 2 ALPPS. PHLF was defined as per the International Study Group of Liver Surgery (ISGLS)’s consensus in 2011 [13]. Long-term study outcomes include length of follow-up, presence of local recurrence, distant recurrence, overall survival (OS) and disease-free survival (DFS). OS and DFS were defined as the percentage of alive or disease-free patients, respectively, at the end of the study, i.e. January 2022.
Treatment protocol
A definitive diagnosis of malignancy was made via CT scan and/or magnetic resonance imaging (MRI) in all patients. Full work-up with CT thorax was completed to ensure no lung metastases. The decision for neoadjuvant chemotherapy prior to ALPPS was made in a tumour board discussion with a panel of medical oncologists, interventional radiologists, hepatopancreatobiliary (HPB) surgeons and gastroenterologists. For patients who received neoadjuvant chemotherapy, there was a minimum of 4 to 6 weeks between the last chemotherapy session and the date of surgery due to the potential liver dysfunction from chemotherapy. This also allows us to establish a baseline ICG-R15 without being confounded by the neoadjuvant chemotherapy status. All patients were prescribed Oral IMPACT (Nestlé, Vevey, Switzerland) three times a day for five days before surgery. Carbohydrate loading was also performed for all patients as part of the ERAS protocol. All patients had pre-operative blood investigations including routine biochemistry, ICG-R15, and CT volumetry. The measurement of ICG-R15 was as such: ICG dye (Verdye, Diagnostic Green GmbH, Aschheim-Dornach, Germany) was administered intravenously at 0.5 mg/kg. Venous blood samples were obtained from the patient at 0 min, 10 min, 15 min and 20 min intervals and sent to the laboratory for analysis of ICG-R15. Interpretation of all the CT volumetry was performed by a single radiologist with special interest in HPB imaging. Prophylactic intravenous cefazolin 2 g was administered 30 minutes prior to skin incision. All patients had ICG-R15 done pre-operatively and after stage 1 ALPPS prior to stage 2 ALPPS. All patients were admitted to high dependency care post-operatively for haemodynamic monitoring in view of surgical complexity and anticipated blood loss. Routine laboratory investigations were performed on post-operative days (POD) 1, 3 and 5, and as per clinical requirements. Interval CT abdomen with volumetry was done about 1 week following stage 1 ALPPS to assess sFLR. All of our included patients had adequate liver hypertrophy following stage 1 ALPPS. In the event of post-operative fever, a full septic workup was performed with two sets of blood culture, chest X-ray with or without CT abdomen and pelvis for further evaluation of possible intra-abdominal collection or an intra-abdominal cause of sepsis. Empiric antibiotics were initiated with piperacillin-tazobactam and one dose of vancomycin as per our institution’s local antibiotic stewardship programme after collection of blood cultures, and adjusted based on culture results. Significant pleural effusion and/or intra-abdominal collection was drained based on clinical discretion via interventional radiology. Following discharge, patients were followed up at 1-2 weeks following discharge, then at 6-monthly intervals. If there were changes in clinical condition or blood abnormalities which were detected, additional follow-ups were given. Interval imaging with CT abdomen and pelvis with triphasic liver or MRI liver was performed at 6 monthly intervals.
Surgical technique
All procedures were performed as open procedures by a single surgeon. For patients with rectosigmoid cancer with liver metastases, high/low anterior resection was performed in the same setting as stage 1ALPPS alongside the colorectal team. ALPPS was performed first in view of the propensity for bleeding and haemodynamic changes requiring need for low central venous pressure (CVP) [14]. Intra-operative hypotension and low CVP may increase the risk of colorectal anastomotic leak [15]. During stage 1 ALPPS, explorative laparotomy was performed to ensure no metastatic disease or progression that precludes resection. Intra-operative ultrasound was performed to confirm location of tumour and resectability. Superficial tumours in the FLR were removed by anatomical or non-anatomical segmental resections. On-table radiofrequency ablation was performed for deep-seated tumours to preserve liver parenchyma when indicated. For all operations in this series, segments I to IV were preserved; however, extended right hepatectomy to include part of segments IVA and IVB or wedge resection of segment IVA or IVB was performed in the presence of tumour metastases in those segments. The right portal vein (PV) (RPV) and hepatic artery (HA) were isolated via an extra-Glissonean approach and only RPV was ligated in continuity during stage 1 ALPPS. However, the left PV was preserved and not slung or mobilised. Segment I branches were preserved, unless there was a need for caudate lobe resection. The right liver lobe was mobilized from the inferior vena cava for the first two cases; however, we did not mobilize the right liver lobe for subsequent cases thereafter to reduce inflammation and adhesions for stage 2 ALPPS. A hanging manoeuvre was performed routinely for the latter 6 cases to facilitate the anterior approach to parenchymal transection. A partial parenchymal dissection (at least 75-80%) was performed for all cases using a bipolar energy device (Caiman, B. Braun, Melsungen, Germany) and the cavitron ultrasonic suction aspirator (CUSA, Valleylab, Boulder, CO, USA). Pringle’s manoeuvre was performed at the surgeon’s discretion when there was significant intraoperative bleeding. A test for bile leakage (white gauze test) was performed. A plastic sheet was used during our initial experience and placed between the split parenchymal surface to prevent adhesion formation for stage 2 ALPPS. Subsequently, TachoSil and Seprafilm (Baxter International Inc, Illinois, United States) were placed to reduce the risk of bleeding and adhesions respectively. The operation was subsequently taken over by the colorectal team for high anterior resection or low anterior resection (LAR) for patients with colorectal cancer with liver metastases. As the purpose of this paper is to describe our initial experience with ALPPS, the details of the colorectal surgery were not described. Defunctioning ileostomy was performed for some patients who had LAR. One or two drains were placed at the resection surface before closing the abdomen.
Stage 2 ALPPS was performed between days 7 and 14 following stage 1 ALPPS. The right HA, right hepatic vein (HV) and right bile duct (BD) were divided. Remaining liver parenchymal bridges were transected. The remaining left lateral lobe was fixed to the anterior abdominal wall to prevent torsion. A drain was placed at the resection surface before closing the abdomen.
Statistical analysis
Study variables were extracted to Microsoft Excel 365 (Microsoft, Washington, United States). Statistical analysis was performed with SPSS version 25.0 (IBM Corp., Chicago, III., USA). Categorical variables were expressed as number (%) and were analysed by χ2 test or Fisher’s exact test if expected cell count < 5. Median (range) were used for all continuous variables. The Wilcoxon signed-rank test was used to compare paired non-parametric variables. Statistical significance was defined as p < 0.05. Due to the short follow-up time and small sample size, OS and DFS were calculated as the percentage of alive or disease-free patients, respectively, at the end of the study. To our knowledge, there has been no study which compared the relationship between the change in ICG-R15 and sFLR following ALPPS. We adopted the linear regression model to compare the change in ICG-R15 and sFLR. Linear regression analysis was similarly used in a previous study comparing ICG-R15 and post-operative bilirubin level, though no significant correlation was observed [16]. In addition, we also used the natural logarithm of the change in ICG-R15 and sFLR as these variables may not follow a linear relationship.
Results
A total of 10 patients were included in this study period. Overall median age was 60.5 years (range 29-69) with male predominance (n = 9/10, 90%). The most common co-morbidities were diabetes mellitus (n = 3/10, 30%) and hyperlipidaemia (n = 2/10, 20%). There was one patient with Child-Pugh A liver cirrhosis. The commonest presenting complaints prior to diagnosis were presence of abdominal pain (n = 5/10, 50%), gastrointestinal bleeding (n = 4/10, 40%) and change in bowel habits (n = 4/10, 40%). The majority of patients received neoadjuvant chemotherapy (n = 7/10, 70%), with a median of 6 cycles (range 5-9). Two patients received CAPOX (oxaliplatin and capecitabine), two patients received FOLFOX (oxaliplatin, fluorouracil and leucovorin) and panitumumab, one received capecitabine, and one received mFOLFOX and cetuximab. Median time from diagnosis to surgery was 6.7 months (range 0.4 to 39.8). We did not have the neoadjuvant chemotherapy regimen for one patient. Table 1 summarizes the baseline clinical characteristics and clinical profile of the patients. The type of resection received during stage 1 ALPPS is also summarized in Table 1.
Table 1
Baseline clinical characteristics of all included patients (N = 10)
[i] All categorical variables were described as n (%) and continuous variables were described as median (range) unless otherwise specified.
[ii] ALP – alkaline phosphatase, ALT – alanine aminotransferase, AST – aspartate amino-transferase, BMI – body mass index, ECOG – Eastern Cooperative Oncology Group, GGT – γ-glutamyl transferase, ICG – indocyanine green, INR – international normalized ratio, RLV – residual liver volume, sFLR – standardized future liver remnant, SLV – standardized liver volume
The most common histology was adenocarcinoma (n = 8/10, 80%) secondary to colorectal origin. Median size of the primary tumour was 5.0 cm (range 2.0-7.0). There was presence of perineural invasion (PNI) and lymphovascular invasion (LVI) in 1 (10%) and 5 (50%) patients respectively. Peri-operative characteristics for both stage 1 and stage 2 ALPPS are summarized in Table 2.
Table 2
Peri-operative characteristics of stage 1 and stage 2 associating liver partition and portal vein ligation for staged hepatectomy (ALPPS)
Median serum total bilirubin on POD5 following stage 1 ALPPS was 13 µmol/l (range 7-24). Table 3 summarizes ICG-R15 and CT volumetry findings pre-operatively and after stage 1 ALPPS. Median time of pre-operative ICG-R15 and CT volumetry prior to stage 1 ALPPS was 9 days (range 2-30) and 32.5 days (range 3-78) respectively. Median time of ICG-R15 and CT volumetry after stage 1 ALPPS was 6.5 days (range 5-9) and 7 days (range 6-8) respectively. The median time of ICG-R15 and CT volumetry between after stage 1 ALPPS and stage 2 ALPPS was 2 days (range 1-3) and 1 day (range 1-4) respectively.
Table 3
Comparison of ICG retention at 15 min and CT volumetry pre-operatively and after stage 1 ALPPS
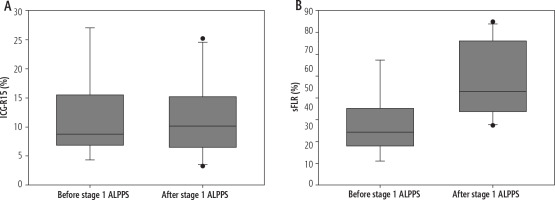
There was a significant increase in median ICG-R15 (Fig. 2A) and sFLR (Fig. 2B) after stage 1 ALPPS compared to pre-operatively: sFLR pre-operatively median 34.4% (range 21.1-67.3) vs. after stage 1 ALPPS 53.0% (range 37.2-84.7), p = 0.012. Linear regression showed no significant correlation between sFLR increase and ICG-R15 (B = 0.26, 95% CI: –0.82, 1.34, p = 0.565). The strength of correlation was also low (R = 0.266, R2 = 0.071). Similarly, linear regression showed no significant correlation between the natural logarithm of sFLR increase and ICG-R15 (B = 0.362, 95% CI: –5.88, 7.61, p = 0.638, R2 = 0.131). Linear regression between percentage change in sFLR increase and ICG-R15 compared to pre-operative values also showed no significant correlation (B = 1.65, R2 = 0.203, 95% CI: –2.11, 5.42, p = 0.310). Logistic regression was not performed to establish a correlation between ICG-R15 or sFLR and incidence of PHLF as there was only one case of PHLF.
Fig. 2
Boxplot showing comparison of (A) ICG-R15 and (B) sFLR before stage 1 ALPPS and after stage 1 ALPPS; ICG-R15 – indocyanine green retention at 15 min; sFLR – standardized future liver remnant; ALPPS – Associating Liver Partition and Portal vein ligation for Staged hepatectomy
There was one 30-day readmission. He was a 51-year old man with extensive bilobar hepatocellular carcinoma (HCC; largest tumour size 6.4 cm) with a background of hepatitis B and Child’s A liver cirrhosis. Prior to his surgery, he was treated with transarterial chemoembolization (TACE) and yttrium-90 radioembolisation (Y-90). Following stage 2 ALPPS, his POD5 INR was 1.9, and total serum bilirubin was 26 µmol/l. He was discharged well on POD20 with no post-operative complications and normalization of INR and bilirubin. He was readmitted 25 days following discharge for decompensated Child-Pugh C liver cirrhosis, presenting with symptomatic ascites requiring therapeutic paracentesis. He subsequently died on POD88 during the same admission secondary to decompensated Child’s C liver cirrhosis. There was one patient with total LOS of 43 days. He was a 66-year old man with stage IV sigmoid colon adenocarcinoma with bilobar liver metastases. Stage 2 ALPPS was performed 7 days after stage 1 ALPPS. During POD3 from stage 2 ALPPS, there was a febrile episode with abdominal pain. CT AP showed a large perihepatic fluid collection with gas locules. He underwent radiologically guided drainage of the perihepatic collection on POD4. He experienced PHLF subsequently on POD7 with uptrending bilirubin, with the highest bilirubin of 210 µmol/l on POD12. He also developed hospital-acquired pneumonia on POD12, for which he was started on intravenous piperacillin-tazobactam. His liver function subsequently improved with normalization of serum bilirubin level, and the peri-hepatic drain was removed on POD21. He was subsequently discharged well and stable on POD43 from stage 1 ALPPS.
Overall post-operative morbidity was 60%. There were 6 patients who had pleural effusion following stage 2 ALPPS, of whom 3 required radiologically guided pleural drainage. There was no 30-day mortality. Six patients (60%) received adjuvant chemotherapy (no. of cycles median 3, range 2-5). Median follow-up period was 11.0 months (range 2.2-73.6). There were 2 patients (20%) with local recurrence, and 3 patients (30%) with metastatic recurrence. For patients who had metastatic recurrence, the site of location was the liver (n = 2), lung (n = 1) and peritoneum (n = 1). Median time to local recurrence and metastatic recurrence was 14.4 months (range 6.9-21.9) and 7.5 months (range 6.9-17.3) respectively. OS and DFS were 50% and 40% respectively.
Discussion
This retrospective case series aims to fill in the gaps to identify whether there is any relationship between volumetric increase versus functional increase of the liver following ALPPS. We were unable to demonstrate any significant relationship between sFLR and ICG-R15. Our study however did show comparable post-operative morbidity and feasibility of ALPPS compared to other low-volume centres.
Post-hepatectomy liver failure is one of the most feared and serious complications following liver resection. To reduce this risk, a minimum of sFLR > 20-25% in healthy patients without underlying liver disease, > 30% in patients with liver steatosis or exposure to chemotherapy, and > 40% in patients with cirrhosis, is required [2]. Unlike conventional TSH, ALPPS allows for rapid liver hypertrophy, reducing the waiting time between the first and second stage of hepatectomy [3]. This reduces the risk of adhesion formation and risk of tumour progression, allowing for higher success of completion of the second stage of hepatectomy [3]. It has been debated whether the rapid increase in volume following stage 1 ALPPS correlates with relative liver function, in view of the high post-operative morbidity associated with ALPPS. A study by Sparrelid et al. on 9 patients with colorectal liver metastases showed a decrease in median ICG-R15 (1 day before stage 1: 9.9% (range 1.2-20.7%), 6 days after stage 1: 7.0% (range 4.2-19.5)), and increase in median sFLR volume percentage by 56.7% (range 32.3-110.4) between 1 day before stage 1 and 6 days after stage 1 [8]. Function of FLR was measured using dynamic planar 99mTc-labelled diethylenetriaminepentaacetic acid galactosyl human serum albumin (GSA) scintigraphy, which showed improvement in median FLR function after stage 1 ALPPS (1 day before stage 1: 1.8%/min/m2, 6 days after stage 1: 2.6%/min/m2). However, the extent of increase in sFLR was significantly greater compared to FLR function increase (sFLR volume increase 56.7%, vs. FLR function increase 28.2%, p = 0.021). They however did not perform any correlation between change in sFLR and ICG-R15. We were unable to validate their findings as use of 99mTc-GSA scintigraphy is not routine prior to liver resection in our institution. While 99mTc-GSA scintigraphy provides information about liver function, it does not accurately distinguish the borders of the liver segment which needs to be resected and preserved, necessitating the need for additional CT imaging. Use of 99mTc-GSA scintigraphy also bears additional costs (range US$564-2,534 in the United States) and has radiation exposure of 90 mGy in the gallbladder and 6.5 mGy in the liver, compared to 10 mGy for CT of the abdomen and pelvis [17]. While their study did show improvement in ICG-R15, both pre-operative and post-stage 1 ALPPS values were < 10% (within the normal range of ICG-R15) and may not be clinically significant [18].
Makuuchi’s criteria serve as a guide for the decision on extent of liver resection in patients with underlying cirrhosis. The normal range of ICG-R15 is 0-10%. In patients with bilirubin ≤ 17.1 µmol/l (1 mg/dl) with ICG-R15 0-10%, patients are eligible for trisectionectomy or hemihepatectomy, while ICG-R15 10-19% limits liver resection to left- or right-sided sectionectomy [18]. Theoretically, rapid liver hypertrophy should result in an sFLR increase with corresponding improvement in liver function (represented by a decrease in ICG-R15) due to liver cellular proliferation [10, 19]. However, we observed an increase in ICG-R15 from 8.8% (range 4.3-27.0) pre-operatively to 10.2% (range 3.2-25.2) following stage 1 ALPPS (median of 6.5 days following stage 1). Kambakamba et al. similarly observed an increase in ICG-R15 from 3.6% (range 2.6-5.7) to 5.4% (range 2.5-10.8) [9]. ICG-R15 is dependent on hepatic blood flow, hepatocellular uptake and biliary excretion [20]. Hyperbilirubinaemia (> 51.3 µmol/l or 3 mg/dl) also increases ICG-R15 by competitive inhibition of ICG transport capacity due to the use of the same carrier system (adenosine triphosphate (ATP), export pump and multidrug resistance associated protein 2 (MRP2)) [21]. While the total bilirubin after stage 1 ALPPS on POD5 in our study was normal (range 7-24 µmol/l) and is unable to explain the increase in ICG-R15, the increase is statistically but not clinically significant (8.8% to 10.2%) and may be due to the small sample size [22]. Nevertheless, this further reinforces the hypothesis that volumetric increase may overestimate the functional increase of remnant liver following ALPPS [6, 8].
We also demonstrated the feasibility and safety of ALPPS in a low-volume centre. Our overall morbidity was 60%, which is comparable to overall minor complications of 59% reported in a meta-analysis on 161 patients who underwent ALPPS [3]. Our results were also similar to results reported by other low-volume centres (overall post-operative complication rate 66.7%) [23]. Thirty-day mortality was also comparable to international reports (range 0-28.7%) [23-26]. In contrast, with a median follow-up of 11 months, we obtained OS of 50% and DFS of 40% respectively by the end of the study, compared to other low-volume centres such as the study by Nadalin et al. (n = 15), who reported a survival rate of 66.7% after a median follow-up of 17 months [23], and Sala et al., who reported OS and DFS of 100% and 80% with a median follow-up of 6.2 months [25]. A possible reason for inferior results reported by our institution may be due to the high incidence of LVI of the primary tumour (50%) reported. LVI is associated with more aggressive tumour behaviour in colorectal cancer (CRC) and serves as a poor prognostic factor for recurrence and mortality in CRC [27]. Additionally, the majority of the included patients received other treatments prior to the offer of ALPPS, e.g. Y-90, TACE and/or neoadjuvant chemotherapy. Hence, there is a considerable delay for ALPPS (median time to surgery from diagnosis was 6.7 months), which may worsen oncological outcomes [28]. Furthermore, not all patients received adjuvant chemotherapy, and of those who received adjuvant chemotherapy, not all completed the course of treatment. Nevertheless, the role of adjuvant chemotherapy is controversial and may not have survival benefits [29, 30].
There are limitations to our study. This is a retrospective case series with a small sample size; however, this is related to the novelty of ALPPS in our low-volume centre. Nevertheless, with a very small sample size and a variety of disease aetiology, neoadjuvant therapy, cirrhosis, and type of resection during stage 1 ALPPS, the results may not be representative. However, the purpose of this study is to highlight the issue of discordance between the change in ICG-R15 and sFLR following ALPPS. We did not obtain ICG-R15 and CT volumetry after stage 2 hepatectomy as this was not part of routine care, and we also did not perform dynamic planar 99mTc-labelled GSA scintigraphy as patients will have to bear additional costs and radiation exposure without additional benefit in clinical care. We were also unable to perform logistic regression to correlate ICG-R15 or sFLR with incidence of PHLF, as there was only one case of PHLF. Data on the type of neoadjuvant treatment received apart from chemotherapy (e.g. Y-90 radioembolization or TACE) were not collected.
Conclusions
This is the first study to investigate a potential correlation between sFLR and ICG-R15, and we did not detect any significant relationship in our centre. This further reinforces the hypothesis that volume increase overestimates the functional increase following ALPPS. This should be validated by prospective studies in large tertiary centres.