Introduction
Portal hypertension (PHT) is a common clinical syndrome defined by increased pressure in the portal venous system. In China, the main cause of PHT is decompensated cirrhosis due to the hepatitis B virus. PHT usually leads to variceal hemorrhage, splenomegaly, and hypersplenism. Variceal hemorrhage is associated with a mortality rate ranging from about 7% to 15% [1]. Patients who have survived variceal hemorrhage still have a high risk of rebleeding (60% in the first year) and a mortality rate of up to 33% [2]. Therefore, surgical treatment for bleeding or prevention of rebleeding is important for recovery of patients. Splenectomy and esophagogastric devascularization (SED), first reported by Yang and Qiu [3] in China, is widely used in China to treat variceal bleeding caused by PHT and has achieved satisfactory results. However, the traditional open procedure is associated with marked tissue trauma and slow postoperative recovery. With the recent technical advances in laparoscopic surgery, laparoscopic SED (LSED) has become a routine procedure for treatment of PHT in many hospitals in China. Notably, LSED is still challenging for many surgeons because of intraoperative bleeding and massive splenomegaly. Fast-track (FT) surgery programs, first described by Bardram et al. [4], have been well developed for management of the immediate perioperative period. The application of fast-track principles greatly accelerates postoperative recovery, reduces the physiologic stress response, lowers the morbidity rate, shortens the hospital stay, and decreases treatment-related costs for surgical patients.
Aim
Up to now, few studies have focused on LSED combined with fast-track principles for treatment of PHT. In the present study, we examined the safety and efficacy of concomitant LSED and fast-track principles with respect to operative outcomes and complications in the treatment of PHT.
Material and methods
Patients
We retrospectively reviewed 145 patients with cirrhotic PHT who underwent LSED in our department from 2016 to 2018. Five patients were excluded due to conversion to open surgery. Finally, 140 patients (92 men and 48 women) who underwent totally LSED were analyzed. All patients showed no intention of liver transplantation after routine preoperative inquiry. Seventy-two patients (47 men and 25 women; mean age: 45.5 ±11.1 years) were treated with a laparoscopic operation in the traditional manner from January 2016 to June 2017. Sixty-eight patients (45 men and 23 women; mean age: 44.2 ±12.4 years) were treated with LSED combined with fast-track principles from July 2017 to December 2018. Ninety-two patients had hepatitis B virus-related cirrhosis, 31 had hepatitis C virus-related cirrhosis, 15 had autoimmune hepatitis cirrhosis, and 2 had unexplained cirrhosis. Fifty-four patients had Child-Pugh class A liver disease and 86 had Child-Pugh class B. Patients with Child–Pugh class C liver disease were excluded because of their poor recovery and high mortality. The indocyanine green 15-minute retention rate was measured for assessment of liver function. A history of variceal hemorrhage (hematemesis and melena) was an absolute indication for a laparoscopic operation. Surgery was also indicated for patients with no history but a high risk of variceal hemorrhage; simultaneous grade III esophageal varices, blue varices, or cherry red spots from bleeding varices diagnosed by endoscopy [5–7]; hypersplenism; and a high hepatic venous pressure gradient of > 12 mm Hg [8].
The study protocol was approved by the ethics committee of TangDu Hospital, and informed consent was obtained from each patient. All procedures were performed in accordance with the relevant guidelines and regulations.
Operative design and procedure
To ensure the most individualized operation and precise medical treatment possible, all patients underwent preoperative multidetector-row computed tomography to evaluate the splenic volume and determine the location of the splenic artery, left gastric vein, and portosystemic collateral vessels. For the splenectomy procedure, each patient was placed in the supine position with the left flank elevated at a 35-degree angle. The locations of the trocars are shown in Photo 1 A. A 12-mm laparoscopic trocar was inserted through an incision beneath the umbilicus (point b in Photo 1 A). Carbon dioxide pneumoperitoneum was established using a high-flow electric insufflator, and four other trocars were inserted under visual control: two 12-mm laparoscopic trocars were separately inserted through the crossover point of the right axillary midline and navel horizontal line (point a in Photo 1 A) and at the level of the lower pole of the spleen (point c in Photo 1 A), and two 5-mm laparoscopic trocars were inserted at a point along the ventrimeson 3 cm below the xiphoid process and through the midpoint between the first location of the 5-mm laparoscopic trocar and the navel (points e and d in Photo 1 A). Point a was used as the laparoscopic observation incision, Points b and c were used as the main operative incisions, and Points d and e were used as the assistant operative incisions. Electrocautery, the LigaSure vessel-sealing system (Medtronic, Minneapolis, MN, USA), hemostatic clamps, and the Endo GIA stapler (Medtronic) were used for vessel disruption.
In most patients, after opening the omental bursa, the splenic artery was separated and ligated (Photo 1 B). Without a blood supply, the spleen volume decreased, making splenic dissection easier to perform and decreasing splenic hemorrhage during the operation. The spleen was transected with an Endo GIA stapler (60–2.5 mm) after all visible splenic attachments had been dissected (Photo 1 C). After dissociating and cutting the left gastric vein at the root with an Endo GIA stapler (45–2.5 mm) (Photo 1 D), the soft tissues and variceal veins were dissected along the greater curvature and lesser curvature of the stomach. The varices along the stomach and lower part of the esophagus (an approximately 5-cm segment) were also resected (Photo 1 E). The spleen was placed in a specimen bag in the abdominal cavity, fragmented within the specimen bag, and removed from the incision in the left lower quadrant (elongated to a 3-cm incision). A drainage tube was placed in the left upper abdomen. The LSED procedure was thus completed (Photo 1 F).
Photo 1
Locations of trocars and key steps in laparoscopic splenectomy and esophagogastric devascularization. A – Trocar locations. (a) Laparoscopic observation incision (below or to the right of the navel). (b, c) Main operative incisions. (d, e) Assistant operative incisions. B – Separation and ligation of the splenic artery. C – Transection of splenic hilar pedicles with the Endo GIA stapler. D – Transection of the left gastric vein at the root with the Endo GIA stapler. E – Esophagogastric devascularization. F – Completion of totally laparoscopic surgery

Perioperative management in FT group and non-FT group
The FT group was managed perioperatively according to the standard fast-track principles. The patients and their families were given detailed information on the perioperative treatment and rehabilitation programs to increase their compliance. Oral carbohydrates were administered in the early morning of the operative day. During the procedure, the patient’s body was kept warm, and warm normal saline was used to wash the abdominal cavity. The infused fluid, especially the crystalloid solution, was controlled to reduce the cardiac burden and tissue interstitial edema. Patient-controlled analgesia was administered by continuous perfusion through a peripheral vein within 48 h postoperatively. The Foley catheter was removed on the morning of the first postoperative day. Gastric decompression was stopped within the first 12 h after surgery. The abdominal drain was removed after 48 h when no further bloody fluid was observed and the amylase concentration of the abdominal drainage fluid was less than three times the serum concentration. The patients were encouraged to sit and engage in in-bed activities the day after the operation, and out-of-bed activities were allowed after the first postoperative day. The patients were encouraged to drink small amounts of water after recovery from anesthesia. When flatus and oral tolerance were reached, a semi-liquid to soft-food diet was gradually introduced. Enoxaparin (0.4 IU twice daily) was subcutaneously injected on postoperative day 2 if no obvious bloody abdominal drainage was observed and the peripheral red blood cell count was stable, which decreased the risk of portal vein thrombosis (PVT) formation. The patients received respiratory physiotherapy and atomizing inhalation of ambroxol during the first 72 h postoperatively. Supplemental plasma or human albumin was administered to maintain the serum albumin concentration at ≥ 30 g/l. A balance was maintained between the liquid intake and output, and furosemide (20 mg/day) was intravenously injected during the first 72 h postoperatively. Somatostatin was used during the first 48 h postoperatively.
Patients in the non-FT group were treated in the traditional manner. They were provided conventional information on PHT. All patients in this group underwent general anesthesia, and a gastric drainage tube, intra-abdominal drain, and urinary catheter were routinely inserted. Postoperative oral intake and rehabilitation were performed in the traditional manner. The details of the perioperative treatments are shown in Table I.
Table I
Peri-operative management compared between the two groups
Operative outcomes and complications
The operative outcomes were the operation time, blood loss volume, postoperative hospital stay, and postoperative mortality and morbidity rates. Any death that occurred in the hospital after the operation was used to calculate the mortality rate. The time to first flatus, first bowel movement, and oral intake and the postoperative hospital stay were recorded. Postoperative complications, including intra-abdominal hemorrhage, gastrointestinal fistula, wound infection, pneumonia, and urinary tract infection, were also assessed.
PVT assessment
A Doppler ultrasound examination of the portal vein was performed preoperatively and postoperatively. The postoperative examination was performed 3, 7, and 14 days after the surgery. PVT was defined as complete or partial (> 50%) occlusion of the portal vein trunk, superior mesenteric vein, or splenic vein. A peripheral venous blood sample was collected from each patient on preoperative day 1 and postoperative days 1, 3, 7, and 14. The D-dimer concentration and platelet count were also determined.
Measurement of inflammatory mediators
The serum levels of C-reactive protein (CRP) and interleukin 6 (IL-6), well-known inflammatory indicators, were detected by enzyme-linked immunosorbent assay kits on preoperative day 1 and postoperative days 1, 3, and 7.
Postoperative follow-up
Long-term follow-up was performed by telephone or at the outpatient department.
Statistical analysis
Continuous data are expressed as mean ± standard deviation and were analyzed by the t-test. Categorical data are expressed as number and percentage and were analyzed by the χ2 test. A p-value of < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS Version 25 (IBM Corp., Armonk, NY, USA).
Results
Patients’ characteristics
The patients’ basic characteristics are shown in Table II. The conversion rate to an open procedure was 3.4% (5/145), 3 of which were due to intraoperative bleeding and 2 were due to abdominal adhesion. No significant difference was found in age, sex, BMI, etiology, indocyanine green 15-minute retention rate, Child-Pugh class, history of variceal hemorrhage, hepatic venous pressure gradient, routine blood examination findings, or serum biochemical parameters between the two groups.
Table II
Characteristics of patients with portal hypertension
Intraoperative and postoperative data
There was no significant difference in the operative time or blood loss volume between the FT and non-FT groups (p > 0.05). According to the fast-track principles, the abdominal drain was removed much earlier in the FT than the non-FT group (p < 0.05). Patients in the FT group had their first bowel movement much earlier than those in the non-FT group (p < 0.05). Similarly, the time to first postoperative flatus was significantly shorter in the FT than the non-FT group (p < 0.05). Patients in the FT group were encouraged to drink and eat earlier. Therefore, under fast-track guidance, the patients in the FT group were discharged from the hospital significantly earlier than those in the non-FT group (p < 0.05) (Table III).
Table III
Intra- and post-operative data
Postoperative complications
Within 30 days postoperatively, one patient in the FT group died of severe intraperitoneal hemorrhage and 1 patient in the non-FT group died of abdominal infection caused by a gastric fistula. The incidence of infectious complications including pneumonia, severe ascites, and urinary tract infection was significantly higher in the non-FT than the FT group (p < 0.05). However, the incidence rates of incision site infection and intra-abdominal infection were not significantly different between the groups. Similarly, the incidence of surgical complications (abdominal bleeding, encephalopathy, pancreatic fistula, and liver failure) was not significantly different between the two groups (Table IV).
Table IV
Postoperative complications and follow-up data
Inflammatory indicators
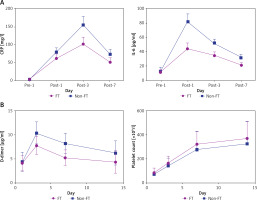
The levels of inflammatory indicators (CRP and IL-6) were not significantly different between the two groups on preoperative day 1. The postoperative CRP and IL-6 levels in both groups were markedly higher than those preoperatively. However, the levels of CRP and IL-6 on postoperative days 1, 3, and 7 were significantly lower in the FT than the non-FT group (p < 0.05) (Figure 1 A).
PVT
As shown in Table IV, although 10 of 68 (14.7%) patients in the FT group and 12 of 72 patients (16.7%) in the non-FT group were diagnosed with PVT by preoperative Doppler ultrasonography and computed tomography examination, the χ2 test showed no significant difference between the two groups (p = 0.875). However, 7 of 58 patients (13.8%) in the FT group and 17 of 60 patients (28.3%) in the non-FT group developed PVT after the operation. The incidence of PVT was significantly lower in the FT than the non-FT group postoperatively (p = 0.028). The D-dimer level was much lower in the FT than the non-FT group on postoperative days 3, 7, and 14 (p < 0.05). The platelet count was significantly higher in the FT than the non-FT group on postoperative days 3, 7, and 14 (Table V and Figure 1 B).
Table V
Portal vein thrombosis
Follow-up
Sixty-two out of the 71 patients (one died after operation was excluded, 87.3%) in the non-FT group were followed up with a mean duration of 19.1 months (1–43 months). In the FT group, the follow-up rate was 89.6% (1 died after operation was excluded, 60/67) and the mean follow-up time was 17.8 months (1.2–28 months). All the patients in both groups are alive. No patient received liver transplantation. During the follow-up period, 7 (11.3%) patients in the non-FT group and 7 in the FT group (11.7%) experienced recurrent esophagogastric variceal hemorrhage. The overall variceal rebleeding rate was 11.5%. No significant difference was found between the two groups (p = 0.948) (Table IV). The esophagogastric variceal rebleeding was controlled by drug, endoscopy or TIPS treatment.
Discussion
For many decades, SED has been widely performed in China and Japan because of its effective control of variceal bleeding, variceal rebleeding, and secondary hypersplenism with little impairment of liver function and a low incidence of encephalopathy [9, 10]. Variceal hemorrhage is currently the major indication for treatment of PHT. Although non-cardioselective β-blockers and variceal band ligation have been recommended for primary prophylaxis of variceal hemorrhage in patients with cirrhosis [11, 12], splenomegaly and hypersplenism cannot be resolved using this technique. Studies have shown that splenectomy can reduce portal venous pressure and improve liver function [13–15]. SED can not only reduce the high risk of variceal bleeding but also lessen the adverse effects of splenomegaly and hypersplenism. LSED has obvious advantages over SED in terms of less trauma and faster recovery [16–18]. Therefore, LSED is an important treatment for patients with a high risk of variceal hemorrhage in our department. The LSED procedure, first applied in our department in 2011, became the first-line therapy for PHT in 2014 after we had accumulated 3 years of clinical experience. The safety and effectiveness of LSED have been confirmed by our own clinical experience and other researchers [10, 19]. Although a limitation of the present study is our retrospective analysis of patients at two different stages, no significant differences in the intraoperative data were found between the two groups, indicating that the operator’s experience and laparoscopic technique were not the beneficial factors in the FT group. Fast-track principles have been extensively used in clinical perioperative management. The widely acknowledged advantages of fast-track principles include acceleration of postoperative recovery, reduction of perioperative morbidity, and shortening of the hospital stay. Most patients with PHT requiring surgery had liver cirrhosis for many years and are in a poor general condition; they often exhibit decompensated liver function, hypoalbuminemia, thrombocytopenia, and coagulation disorders. Less surgical trauma and more rapid recovery from surgery appear particularly important for these patients. Therefore, LSED combined with fast-track principles may be a suitable treatment method. Research has shown that fast-track principles reduce the hospital stay from 8.4 to 6.3 days on average. Significantly improved postoperative recovery with respect to toleration of a liquid diet, first flatus, and first defecation has been observed. The incidence of pneumonia, urinary tract infection, and the inflammatory response is significantly reduced with fast-track principles, and a decreased incidence of PVT has been detected.
Gastrointestinal dysfunction frequently occurs in postoperative patients and can intensify the therapeutic efforts and prolong the hospital stay [20–22]. Many Chinese people have long held a misconception that good recovery requires long-term bed rest. Operation-related stress is also not conducive to postoperative recovery. Thus, high-quality preoperative education is necessary to rectify inaccuracies and relieve patients’ anxieties. When sufficient and proper preoperative education is provided, patients’ cooperation and motivation increase [23]. Pain management is also very important in the FT protocol. Proper postoperative analgesia can not only decrease the pain-related stress response but can also eliminate the discomfort of early ambulation [24]. Opioids are a commonly used analgesic after surgery, but they may delay gastrointestinal mobility and even cause nausea and vomiting, which may slow recovery [25]. Because LSED is a minimally invasive approach that induces less pain than traditional open surgery, postoperative analgesia was administered in this study only when the pain could not be tolerated. Therefore, with the help of comprehensive treatment guidance, patients’ compliance with out-of-bed activities at an early stage was significantly improved in the FT group. Beyond that, placement of a long-term indwelling gastric tube is a risk factor for nausea and vomiting. In many operations, a nasogastric tube is not yet routinely placed in accordance with the fast-track policy. However, SED may lead to ischemia of the gastric fundus, resulting in a gastric fistula. Therefore, we routinely placed a nasogastric feeding tube to relieve intragastric pressure but terminated it within the first 12 h postoperatively in the FT group. With sufficient preoperative education, adequate pain management, and early mobilization, the patients in the FT group showed earlier return of intestinal function postoperatively.
Many studies have shown that the fast-track protocol has positive effects on the human immune system and decreases the perioperative stress response to surgical trauma [26]. The normal inflammatory response, first proposed by Cuthbertson and Tilstone [27], may play a vital role in the process of wound healing and infection resistance; however, it may also lead to pain, emergence of opportunistic infections, fatigue, and organ dysfunction. Multiple factors determine the severity of the postoperative inflammatory reaction. Early recovery of gastrointestinal activity maintains the intestinal mucosal barrier function and can help to maintain the immune function. Compared with open surgery, laparoscopic minimally invasive surgery and less operative bleeding are both beneficial for reduction of the inflammatory reaction. Proinflammatory cytokines such as CRP and IL-6, which are triggered by the inflammatory reaction [28], are commonly used as inflammatory indicators. IL-6 is reportedly associated with the severity of surgical trauma [29]. IL-6 can also stimulate the liver to synthesize CRP and enhance the inflammatory response [30]. In the present study, we found no significant difference in the preoperative levels of inflammatory indicators between the two groups. Both the IL-6 and CRP levels increased significantly after the operation. However, the IL-6 and CRP levels were significantly lower in the FT than the non-FT group on postoperative days 1, 3, and 7 (p < 0.05). All patients in both groups underwent totally laparoscopic surgery. No statistically significant difference was found in the operation time or intraoperative blood loss volume between the two groups. These findings indicate that rapid rehabilitation may play an important role in reducing the postoperative inflammatory reaction.
PVT is a common and important complication of PHT, especially in patients undergoing splenectomy and azygoportal disconnection. One study showed that the incidence of PVT after laparoscopic surgery was higher than that after open surgery, although the rate of occlusion of the main trunk of the portal vein was similar [31]. PVT may lead to liver dysfunction, ascites, intestinal edema, and an increased risk of variceal bleeding [32]. Therefore, reducing the incidence of PVT is conducive to reducing postoperative complications. The safety of anticoagulation in patients with PHT, especially perioperatively, has been debated for many years because of the risk of bleeding. However, increasingly more studies have shown the safety and effectiveness of anticoagulation in patients with PHT [33, 34]. In our clinical experience, laparoscopic surgery with meticulous manipulation and adequate intraoperative hemostasis is important for postoperative anticoagulation. In the present study, 3 of 58 patients (5.17%) in the FT group stopped anticoagulation therapy because of abdominal bleeding (data not shown). Although two patients in the FT group needed emergency surgery, the operation confirmed that the abdominal bleeding had been caused by dropping of a hemostatic clip and poorly executed suturing of the subumbilical trocar incision (the periumbilical abdominal veins are enlarged in patients with PHT). In the remaining patient (1 of 58 patients, 1.72%), the abdominal hemorrhage was stopped after termination of the anticoagulation therapy. Therefore, our study demonstrated the safety of anticoagulation therapy. Additionally, the incidence of PVT in the FT group significantly decreased with anticoagulation therapy. A reduced incidence of PVT may contribute to reductions in gastrointestinal edema, gastrointestinal bacterial translocation, and ascites, thereby promoting gastrointestinal function recovery and lessening postoperative inflammation.
Infection is a risk factor for postoperative morbidity and mortality [35]. Studies have shown that the incidence of infection decreases with use of the FT protocol [36]. In our study, the rates of urethral infection and pneumonia were significantly reduced in the FT group, but no significant difference in the incidence of abdominal or incision infection was found; this might have been due to the minimal invasiveness of laparoscopic surgery.