Introduction
A perianal fistula, also known as a fistula-in-ano, is an abnormal hollow tract or tunnel lined with granulation tissue that connects the main hole inside the anal canal to a secondary hole on the perianal skin. The annual incidence of perianal fistulas ranges from 1.1 to 2.2 per 10,000 individuals [1, 2]. It is one of the most prevalent diseases negatively affecting the quality of life of patients. In particular, complex perianal fistulas, which are a category of perianal fistulas, are characterized by a high occurrence rate and are difficult to treat using current surgical techniques. They often feature several external openings, such as extrasphincteric, suprasphincteric, highly intersphincteric, or highly trans-sphincteric, and are connected to an abscess, rectovaginal fistula, or anorectal stenosis [3, 4]. Treatment goals for complex perianal fistulas include stopping the fistula from draining, alleviating symptoms, preventing complications or fecal incontinence, and achieving permanent closure [5]. The primary clinical treatment methods for complex perianal fistulas include traditional medications (such as antibiotics and immunomodulators), which could improve the symptoms but cannot completely heal the complex perianal fistulas; anti-tumor necrosis factor (TNF) agents, such as certolizumab, infliximab, and adalimumab; and surgical treatments and fecal diversion. However, most current therapies are associated with a high recurrence rate, and it is difficult to maintain long-term fistula closure [6-8]. Thus, innovative therapies for complex perianal fistulas are urgently needed. Complex perianal fistula, a chronic recurrent disease mediated by the immune system, presents a major challenge to fistula closure and fibrosis, and mesenchymal stem cells (MSCs) should be considered as a prospective treatment option. MSCs could offer a highly promising way for the treatment of complex perianal fistulas, primarily due to their potent anti-inflammatory and immunomodulatory properties [9, 10]. The advantage of MSCs is their ability to be delivered directly to fistula tracts. This targeted delivery method not only increases the concentration of therapeutic cells at the site of injury but also enhances the interaction between MSCs and local cells, fostering a more conducive environment for tissue repair and regeneration. Furthermore, MSCs are widely recognized as one of the most extensively studied and utilized types of multipotent stem cells in the field of regenerative medicine. Their versatility and compatibility make them suitable candidates for various therapeutic applications, including fistula treatment [9]. A notable feature of MSCs is their low immunogenicity, allowing them to bypass barriers posed by the human leukocyte antigen complex. This immunological advantage means that MSCs are less likely to trigger an immune response or cause immunological rejection after transplantation, increasing their efficacy and safety as a therapeutic option [11]. Several studies have provided robust evidence supporting the positive impact of MSCs on complex perianal fistulas. These studies have demonstrated improvements in fistula closure rates, reduced recurrence rates, and enhanced overall patient outcomes following MSC therapy [12, 13]. However, considering their long-term recurrence, we believe that the effects of long-term treatment are an important aspect that needs to be addressed. Accordingly, we conducted this systematic review and meta-analysis to assess the long-term effectiveness of MSCs in the treatment of complex perianal fistulas.
Methods
Literature search
A systematic review and meta-analysis was performed to investigate the long-term effects of MSCs on perianal fistulas. Relevant studies were identified by conducting a thorough search of PubMed and other electronic databases (Medline, Embase [Ovid], Web of Science, and the Cochrane Central Register of Controlled Trials) for those published up to April 2023. The search was restricted to clinical studies published in English. The following search terms were used: (“mesenchymal stem cells” OR “mesenchymal stromal cells” OR “stem cells” OR “stroma cells”) AND (“inflammatory bowel disease” OR “Crohn’s disease”) AND (“fistula”) OR (“perianal fistula”) OR (“complex perianal fistula”), and any other abbreviations that may be relevant.
Study eligibility
The articles retrieved from the search were individually reviewed and the reference lists of published trials and conference abstracts were assembled for potentially eligible studies. The analysis includes studies that met the following criteria: clinical trials involving patients diagnosed with perianal fistula, treatment with MSCs, age > 18 years, reported outcomes of efficacy and safety, published in English. Studies not published in English, those that contained insufficient information or data, and those reporting follow-up < 8 weeks were excluded.
Data extraction and statistical analysis
Each study was evaluated by two researchers, with any disagreements resolved by a third researcher. The title, author information, publication year, study origin, demographic details (sex, age, country, and sample size), and measurable results were all independently collected by researchers from the articles listed above. These discrepancies have been resolved. The Newcastle-Ottawa Scale (NOS) was used to rate the quality of each study. “High quality” trials received scores > 6. After the two evaluators rated the quality of the studies independently, a third researcher assessed disparities. Stata version 13.0 (Stata Corp., College Station, TX, USA) was used to perform statistical analyses. The effect size was measured using a confidence interval (CI) of 95% and the standard deviation (SD) of the mean. Heterogeneity of the studies was evaluated using the Q test and I2 analysis. The source of heterogeneity was identified using meta-regression analysis. Egger’s linear regression test and Begg’s funnel plot were used to examine publication bias. Differences with p < 0.05 were statistically significant.
Results
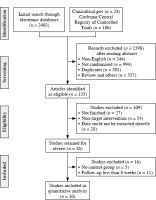
The electronic literature search yielded 2533 potentially relevant studies. Thorough assessment of titles and abstracts of 2398 articles yielded 557 reviews and references, 346 published in languages other than English, and 501 duplicates. One hundred and nine studies were excluded from the analysis, including 27 unfinished studies, non-target inventions (n = 54), studies containing data/information that could not be effectively extracted (n = 28), and those that had a follow-up < 8 weeks (n = 11) or had no control group (n = 5) (Fig. 1).
The final meta-analysis ultimately included 10 studies from all the studies analyzed, including a total of 1049 patients and published between 2009 and 2022. There were between 5 and 107 patients per sample (median, 25). Patients included in the analysis ranged in age from 24.4 to 50.85 years (mean [±SD] age, 40.6 ±6.85 years). The follow-up periods of the studies ranged from 24 to 156 weeks. Seven studies were randomized controlled trials (RCTs) and three were retrospective studies. The primary outcome in all investigations was clinical response (CR). Eight studies reported adverse events (AEs) for safety assessment. Seven trials investigated treatment using adipose tissue-derived MSCs and three used bone marrow-derived MSCs. Table 1 summarizes relevant information regarding all of the included studies [13-22].
Table 1
Characteristics of all included studies
| Study | Disease type | Type of study | No. of patients | Age Mean (SD) | Gender (M/F) | Intervention | Follow up | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | C | T | C | T | C | Type of MSCs | Dose of MSCs | |||||
| Garcia-Olmo 2009 [14] | CD | RCT Phase II | 24 | 25 | 42.64 | 43.99 | 10/14 | 14/11 | Allogeneic adipose-derived stem cells | 2-6 × 107 cells | 48 weeks | CR, SF-12, SAE |
| Guadalajara 2012 [22] | CD, non-CD | Retrospective study | 21 | 13 | 41 (9.7) | 40.0 (9.3) | 8/13 | 7/6 | Adipose-derived stem cells | NR | 240 weeks | CR, AE |
| Herreros 2012a [13] | non-CD | RCT Phase III | 64 | 59 | 49.38 (11.39) | 50.85 (12.51) | 47/17 | 44/15 | Autologous adipose-derived stem cells | T1group: 2 × 107 C group: fibrin glue + saline solution | 48 weeks | CR, SF-12, SAE |
| Herreros 2012b [13] | non-CD | RCT Phase III | 60 | 59 | 47.24 (12.27) | 50.85 (12.51) | 36/24 | 44/15 | Autologous adipose-derived stem cells | T2 group: 2 × 107 C group: fibrin glue + saline solution | 48 weeks | CR, SF-12, SAE |
| Molendijk 2015a [15] | CD | RCT | 5 | 6 | 40.4 (4.6) | 37.3 (3.6) | 4/1 | 3/3 | Allogeneic bone marrow derived mesenchymal stromal cells | T1 group: 1 × 107 C group: saline solution | 24 weeks | CR, MRI, PDAI, CDAI, IBDQ, CDEIS, SESCD, SF-36, CRP, IL-8, IL-1β, IL-6, IL-10, TNF, IL-12p70, SAE |
| Molendijk 2015b [15] | CD | RCT | 5 | 6 | 40.8 (1.7) | 37.3 (3.6) | 4/1 | 3/3 | Allogeneic bone marrow derived mesenchymal stromal cells | T2 group: 3 × 107 C group: saline solution | 24 weeks | CR, MRI, PDAI, CDAI, IBDQ, CDEIS, SESCD, SF-36, CRP, IL-8, IL-1β, IL-6 and 10, TNF, IL-12p70, SAE |
| Molendijk 2015c [15] | CD | RCT | 5 | 6 | 33.4 (5.2) | 37.3 (3.6) | 1/4 | 3/3 | Allogeneic bone marrow derived mesenchymal stromal cells | T3 group: 9 × 107 C group: saline solution | 24 weeks | CR, MRI, PDAI, CDAI, IBDQ, CDEIS, SESCD, SF-36, CRP, IL-8, IL-1β, IL-6 and 10, TNF, IL-12p70, SAE |
| Panés 2016 [16] | CD | Phase III RCT | 107 | 105 | 39.0 (13.1) | 37.6 (13.1) | 60/47 | 56/49 | Allogenic adipose-derived mesenchymal stem cells | 5 × 106 cells/ml, 24 ml | 24 weeks | MRI, PDAI, CDAI, IBDQ, van Assche score, IgG, AE, SAE |
| Panés 2018 [21] | CD | Phase III RCT | 107 | 105 | 39.0 (13.1) | 37.6 (13.1) | 60/47 | 56/49 | Allogenic adipose-derived mesenchymal stem cells | 5 × 106 cells/ml, 24 ml | 52 weeks | IBDQ, PDAI, CDAI, TNF, MRI, TEAE |
| Zhou 2020 [17] | CD | RCT | 11 | 11 | 24.4 (5.0) | 24.9 (5.4) | 11/0 | 10/1 | Allogenic adipose-derived mesenchymal stem cells | 5 × 106 cells/ml, dosage based on diameter and length of fistula | 48 weeks | CR, MRI, ultrasonography, CDAI, PDAI, IBDQ, VAS, Wexner score, CRP, ESR, FC, SAE |
| Ascanelli 2021 [18] | non-CD | RCT | 58 | 58 | 50.41 (14.2) | 50.41 (14.8) | 21/37 | 24/34 | Autologous centrifuged adipose tissue | NA | 24 weeks | DSA, HLA-I, CD55, 46, 59, AE, SAE |
| Garcia-Olmo 2022 [19] | CD | Phase III RCT | 25 | 15 | 38.6 (14.4) | 42.7 (14.8) | 14/11 | 8/7 | Allogenic adipose-derived mesenchymal stem cells | 5 × 106 cells/ml, 24 ml | 104 weeks | TEAEs CR |
| Panés 2022 [20] | CD | Retrospective study | 43 | 46 | 42.1 (13.7) | 37.7 (13.5) | 24/19 | 25/21 | Allogenic adipose-derived mesenchymal stem cells | 5 × 106 cells/ml, 24 ml | 156 weeks | SAEs CR |
[i] RCT – randomized controlled trials, PE – physical examination, MRI – magnetic resonance imaging, CDAI – Crohn’s Disease Activity Index, PDAI – Perianal Disease Activity Index, CRP – C-reactive protein, TGF-β – transforming growth factor β, DSA – donor-specific antibodies, VSA – pain scores with visual analog score, ESR – erythrocyte sedimentation rate, FC – fecal calprotectin, TEAEs – treatment-emergent adverse events, IBDQ – Inflammatory Bowel Disease Questionnaire, TNF – tumor necrosis factor, CR – clinical response, CD – Crohn’s disease
Clinical response
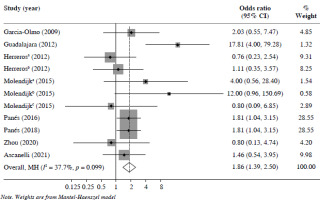
24 weeks’ follow-up: Eight articles reported on CR with a follow-up of 24 weeks. The results demonstrated that the treatment group benefited more from MSC treatment than the control group (odds ratio [OR] = 1.86, 95% CI: 1.39-2.50, p < 0.05) (Fig. 2).
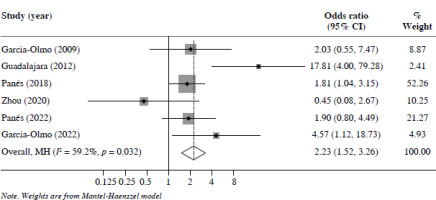
48 weeks’ follow-up: Six studies reported CR at 48 weeks’ follow-up. The MSC group exhibited significantly improved outcomes compared with the control group (OR = 2.23, 95% CI: 1.52-3.26, p < 0.05) (Fig. 3).
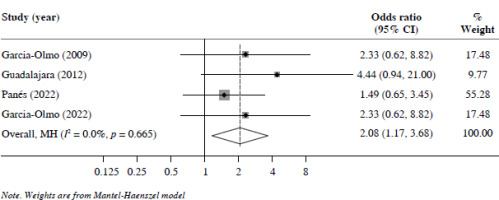
96 weeks’ follow-up: Four studies reported CR at 96 weeks’ follow-up. Overall, the MSC treatment group exhibited better CR than the control group (OR = 2.08, 95% CI: 1.17-3.68, p < 0.05) (Fig. 4).
Crohn’s Disease Activity Index
24 weeks’ follow-up: Five of the studies included in this analysis reported Crohn’s Disease Activity Index (CDAI) at 24 weeks’ follow-up, and the outcomes revealed that there was no significant difference between the MSC and control groups (standardized mean difference [SMD] = 0.97, 95% CI: –0.02-1.97, p = 0.06) (Fig. S1).
52 weeks’ follow-up: Two of 10 studies investigated CDAI with 52 weeks’ follow-up, and there was no significant difference between the treatment and control groups (SMD = 0.51, 95% CI: –0.55-1.56, p = 0.35) (Fig. S2).
Perianal Disease Activity Index
24 weeks’ follow-up: Two of the 10 studies researched Perianal Disease Activity Index (PDAI) with 24 weeks’ follow-up. Overall results revealed no significant difference between the MSC and control groups (SMD = –0.07, 95% CI: –0.66-0.51, p = 0.80) (Fig. S3).
52 weeks’ follow-up: Two of 10 studies researched PDAI with 52 weeks’ follow-up; overall, no significant difference was found between the MSC and control groups (SMD = –0.2, 95% CI: –0.47-0.06, p = 0.13) (Fig. S4).
Safety – adverse events
Eight articles reported AE data and the results of the analysis revealed no significant differences between the MSC and control groups (risk ratio [RR] = 0.87, 95% CI: 0.69-1.10, p = 0.24) (Fig. S5).
Subgroup analyses
Subgroup analyses according to the following factors were performed: treatment cell type (adipose tissue- derived MSCs [vs.] bone marrow-derived MSCs); disease type (Crohn’s vs. non-Crohn’s); publication year (before 2018 vs. after 2018); sample size (< 50 vs. ≥ 50); and study quality (NOS score < 7 vs. ≥ 7) (Table S1).
Subgroup analysis based on treatment cell type
A subgroup analysis based on treatment cell type was performed with the included studies divided into two groups (i.e., adipose tissue-derived MSCs vs. bone marrow-derived MSCs). Both adipose tissue-derived and bone marrow-derived MSCs affected CR at 24-, 48-, and 96-week follow-ups compared with the control group (Figs. S6, S7, and S8). Meanwhile, bone marrow-derived MSCs lowered the CDAI after 24 weeks’ follow-up (Fig. S9).
Subgroup analysis based on disease type
The included studies were split into three subgroups following stratification according to disease type, as follows: patients with Crohn’s, non-Crohn’s, and Crohn’s and non-Crohn’s combined. The MSC treatment group exhibited significantly improved 24-week follow-up CR in those with Crohn’s disease (Fig. S10). Furthermore, MSC treatment considerably increased 48-week follow-up CR in the Crohn’s and combined Crohn’s and non-Crohn’s disease groups (Fig. S11).
Subgroup analysis based on study quality
Subgroup analysis was conducted according to study quality divided into two subgroups: NOS score < 7 and ≥ 7. The results revealed that in the subgroup of score ≥ 7, 24 weeks’ follow-up CR was improved considerably (Fig. S12). Moreover, at 48 weeks’ follow-up, CR was significantly enhanced in both subgroups (Fig. S13). It was also found that in the subgroup of score ≥ 7, the treatment group could decrease the RR of AEs; however, in the subgroup of score < 7, the RR for AEs was higher than that of the control group (Fig. S14).
Subgroup analysis based on sample size
A subgroup analysis was performed in accordance with sample size, and the studies were separated into two subgroups: those with < 50 and those with ≥ 50 patients. The results indicated that, in both subgroups, MSC treatment increased the CR effect at the 24- and 48-week follow-ups (Figs. S15 and S16). In the < 50 patient subgroup, MSC treatment significantly improved 96-week follow-up CR; however, for the ≥ 50 patient subgroup, there was no clear evidence that 96-week follow-up CR could be enhan- ced after MSC treatment (Fig. S17).
Subgroup analysis based on publication year
Based on sample size, the studies were separated into two subgroups: those published before 2018 and those published after 2018. Follow-up CR at 24 and 48 weeks increased considerably in both subgroups (Figs. S18 and S19). Regarding CR at the 96-week follow-up, MSC therapy demonstrated better efficacy in the subgroup of studies published before 2018 (Fig. S20). In the subgroup of publications published before 2018, treatment with MSCs lowered the RR for AEs (Fig. S21).
Discussion
The present study focused on the effectiveness of long-term MSC treatment for complex perianal fistulas. We included both adipose tissue-derived and bone marrow- derived MSCs in the assessment and collected follow-up data for 24 to 96 weeks. The results revealed that MSCs improved CR from 24 to 96 weeks of follow-up. How- ever, the RR for AEs did not increase. Several studies have demonstrated the effects of MSCs on complex perianal fistulas [13, 23-26]. Our results are consistent with those reported by Panés et al. and Guadalajara et al., who focused on the long-term efficacy of MSCs [21, 22]. Because high recurrence rates are a feature of a complex perianal fistula, we believe that assessing the long-term effect of treatment is crucial. Although many studies have evaluated the effects of MSCs on complex perianal fistulas, no study has systematically assessed their long-term efficacy. To our knowledge, this is the first study to investigate the long-term follow-up period of MSC treatment for complex perianal fistulas. We excluded short-term follow-up trials (< 24 weeks) and included only long-term follow-up trials. Considering the high heterogeneity in our results, we conducted a subgroup analysis. However, our analysis failed to identify any sources of heterogeneity. It has been established that complex perianal fistulas are always associated with Crohn’s disease; moreover, complex perianal fistula is not limited to adults, but also occurs among infants and adolescents [27]. Among adolescents, the prevalence of perianal disease ranges from 8% to 26% [28, 29]. Among the current treatment options, surgical methods are optimal; however, surgical treatment is associated with poor healing and a high recurrence rate [30]. Traditional medications – anti-inflammatory bowel disease drugs – showed no significant advantages compared to the control group [31, 32]. According to research, immunomodulators appear to be ineffective at promoting the healing of fistulas [33]. MSCs are an innovative treatment for complicated perianal fistulas, and research investigating MSCs for complex perianal fistulas has increased in recent years. MSCs exert their immunomodulatory effects through various mechanisms, including their ability to migrate towards areas of inflammation or tissue damage, release anti-inflammatory molecules such as interleukin 10, hepatocyte growth factor, and transforming growth factor β1 [34], and engage in paracrine signaling with neighboring cells to uphold a localized anti-inflammatory environment. Through this process, MSCs facilitate the upregulation of a CD4+ T-cell subset (known as regulatory T cells) [35-37]. Meanwhile, MSCs possess immunomodulatory properties that inhibit the activation, proliferation, differentiation, and maturation of immune cells, as well as the capacity to differentiate into numerous mesodermal cell lineages. Given all these characteristics of MSCs, it is reasonable to consider them as a potential treatment option. In this study, we collected adipose tissue and bone marrow-derived MSCs. According to a previous study [38], MSCs generated from adipose tissue may be more effective than bone marrow MSCs in reducing immunological responses in vitro. However, based on our subgroup analysis, there were no significant differences between adipose tissue-derived and bone marrow-derived MSCs. Both can improve CR at 24 to 96 weeks’ follow-up. The difference in results may be due to the fact that only three trials used bone marrow-derived MSCs. Because of the immunosuppressive properties of MSCs, the risk for malignancy should be considered in clinical applications. In our study, 8 of the 10 studies reported adverse events, and no cancer-related AEs were reported. According to a 4-year follow-up study, there was no carcinogenic risk associated with MSC treatment [22]. The clinical evidence of its safety is insufficient; more clinical trials are needed to determine the safety of MSCs, and specific guidelines for the application of MSCs to complex perianal fistulas should be established. Treatment with MSCs is effective for the long-term treatment of complex perianal fistulas, particularly among infants and adolescents. Since drugs for adolescents are limited and MSCs may not pose a serious risk for them, MSCs are a option for adolescents with complex perianal fistulas.
Limitations
First, the studies included in our analysis were not RCTs. Two retrospective studies were included in this meta- analysis. Compared with all RCTs, the evidence may be insufficient. Second, we did not find a source of high heterogeneity despite using a fixed-effects model for the subgroup analysis. Third, the safety data for long-term MSC treatment of complex perianal fistulas are insufficient, and more trials addressing this important issue should be performed.
Conclusions
Long-term treatment with MSCs improved CR but had no obvious effects on CDAI and PDAI at the 24- and 52-week follow-ups. Based on these results, MSCs represent a potentially safe option for the therapy of complex perianal fistulas. Nevertheless, more clinical trials should be carried out to verify the effectiveness and safety of MSCs. This could benefit not only adult patients, but also infants and adolescents.