Introduction
The World Health Organization’s (WHO) definition of obesity is ‘an abnormal or excessive fat accumulation that presents health-risks’. According to the WHO, obesity is classified by the internationally accepted body mass index (BMI) [1]. This medical condition has reached epidemic proportions worldwide, accounting for more than 1.9 billion overweight and approximately 650 million obese adults [2]. There is greater morbidity and mortality in patients with severe obesity, especially classes II (BMI 35.0 to 39.9) and III (BMI greater than 40). In addition, obesity is a recognized risk factor for the development of comorbid conditions such as cardiovascular disease, type 2 diabetes mellitus, malignancy, asthma, osteoarthritis, chronic back pain, obstructive sleep apnoea, non-alcoholic fatty liver disease, and gallbladder diseases [3].
Achieving weight loss (WL) through a healthy lifestyle seems to be an ideal solution. However, surgical management remains the most successful and effective method for patients with excessive adipose tissue (especially class II and III) [4]. Today, Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and adjustable gastric banding are the most popular and commonly performed bariatric surgeries (BS) [5]. Sleeve gastrectomy is a safe and efficient procedure for the treatment of obesity and its related comorbid disorders [6]. Laparoscopic sleeve gastrectomy (LSG) has become a popular WL surgery over the last 10 years and has emerged as a proper treatment option for morbidly obese patients [7]. In this laparoscopic method, a large part of the stomach, which accounts for the regulation of appetite, is resected. Laparoscopic sleeve gastrectomy is a minimally invasive procedure and is considered less technically challenging than laparoscopic RYGB because it does not require a gastrointestinal anastomosis or intestinal bypass [8]. Short-term outcomes of SG – in regard to WL – are comparable to RYGB. However, the effect of SG on nutritional deficiencies over time is currently under study [9]. According to the literature, there is a high prevalence of micronutrient deficiency among obese patients prior to bariatric surgery. These nutrient deficiencies may increase or occur de novo after the bariatric procedure [10]. The mechanism of their development differs according to surgical procedures and individuals. Vitamin B12, folic acid, iron, calcium, and thiamine are some of the most common nutritional deficiencies among patients who undergo bariatric surgery for weight loss. However, there are not many specific reports of nutritional deficiencies among SG patients. Patients undergoing SG are at risk of these deficiencies because of decreased hydrochloric acid and intrinsic factor secretion, reduced intake, poor food choices, postoperative vomiting and nausea, as well as food avoidance due to intolerance [9]. The purpose of this study was to present long-term WL outcomes as well as to determine the amount of preoperative micronutrient deficiencies in bariatric patients who undergo SG, and to assess the evolution of nutritional status during a 6-year postoperative period.
Material and methods
Between January 2005 and December 2010, we collected patients who were morbidly obese and treated with laparoscopic LSG as a primary bariatric surgery at a single centre (“Holy Mary the Help” General University Hospital of Patras), a governmental tertiary hospital in Greece, covering a population of approximately 1.5 million people. Yearly follow-up is provided and suggested to all patients undergoing bariatric surgery at our institution.
Inclusion criteria for the SG were BMI ≥ 40 kg/m2 without associated comorbid conditions or BMI > 35 kg/m2 with at least 1 comorbidity such as diabetes, heart disease, high blood pressure, or severe sleep apnea, and unsuccessful attempts at medical treatment. Exclusion criteria were psychiatric illness or substance abuse.
All patients were informed in detail of the procedure, follow-up, advantages, and complications. Ethical approval was obtained from the medical research ethics committee. Due to the retrospective nature of this study, informed consent from the Institutional Review Board of the “Holy Mary the Help” General University Hospital of Patras was not required. We confirmed that all methods were performed in accordance with the approved guidelines and regulations. The data were collected from the hospital registry and patient files.
Preoperative characteristics included age, gender, height, weight (WT), and BMI. The mean excess body weight loss (EWL) was calculated as (WT before – WT after) × 100/(WT before – ideal body WT). Ideal body WT = BMI of 25 kg/m2 [6]. Patients were followed up post-operatively on a yearly basis by bariatric surgeons. Nutritional status was assessed by routine laboratory tests at each follow-up visit. The 6-year follow-up data were collected via interview.
Nutrient deficiencies were diagnosed by blood analysis. The evolution of nutritional status included haemoglobin, iron, ferritin, folic acid, vitamin B12, magnesium, phosphorus, and parathormone (PTH). Laboratory values were regarded as deficient when they did not meet the reference values determined by our clinical laboratory. Deficiencies that were found either pre- or postoperatively were supplemented. Only patients who completed a preoperative blood test and at least 1 yearly follow-up post-surgery were included for this study. Patients who did not complete blood testing or those who had a revision to RYGB were excluded from the study.
Statistical analyses were done using SPSS for Windows 10. Student’s t-test for normally distributed variables, U Mann-Whitney test for skewed variables, χ2 test, and Fisher’s exact tests were used to compare results between groups. A p-value < 0.05 was considered statistically significant.
Results
During the study period, 209 patients underwent LSG. The characteristics of the study group are shown in Table 1. Of the patients, 54 (25.8%) were men and 155 (74.2%) were women; overall, the mean age was 34.4 ±10 years. The mean weight of participants was 123.1 ±15.9 kg and mean BMI was 43.2 ±2.8 kg/m2. 197 out of 209 (94.3%) patients completed a follow-up of 1 year. Sixty patients (28.7%) attended the 6-year follow-up. Fifty-eight out of 60 patients who attended the 6-year follow-up were able to attend all 6 visits. The one missed the 2-year follow-up, while the other one missed the 5-year follow-up (Table 2). Marked WL was observed during the first year in all patients, achieving a mean BMI of 26.3 kg/m2 with a mean % EWL of 80.9 ±15.6. Six years after surgery, the BMI of patients decreased from an average of 43.2 ±3.5 to 29.8 ±5 (p < 0.001). Patients’ BMI as well as % EWL during the 6-year postoperative period are presented in Figure 1.
Fig. 1
Evolution of body mass index and percent of excess weight loss during the six-year follow-up
BMI – body mass index, EWL – excess weight loss
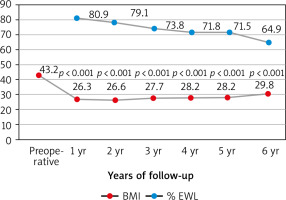
Table 1
Characteristics of the study population (n = 209)
| Factor | |
|---|---|
| Male, n (%) | 54 (25.8) |
| Female, n (%) | 155 (74.2) |
| Age (years) | 34.4 ±10.0 |
| Weight (kg) | 123.1 ±15.9 |
| Height (cm) | 168.5 ±8.8 |
| BMI (kg/m2) | 43.2 ±2.8 |
| IBW (kg) | 63.0 ±7.4 |
| EBW (kg) | 60.0 ±10.2 |
Table 2
Weight loss results of laparoscopic sleeve gastrectomy over time
The mean haemoglobin (Hb) level was 13.7 pg/ml preoperatively, whereas the mean Hb level was 13.4 pg/ml 1-year post surgery and 13.0 pg/ml 6 years after SG (p = 0.038, p = 0.003, respectively). Table 3 shows the mean values of micronutrients: haemoglobin, iron, ferritin, folic acid, vitamin B12, magnesium, and PTH before and after surgery.
Table 3
Changes in absolute values in micronutrients following sleeve gastrectomy
Prior to surgery, 36 out 209 (17.2%) of patients had anaemia. Postoperatively, the deficiency of haemoglobin worsened (27.4% 1-year post-surgery, and 36.7% 6 years after SG; p = 0.014, p = 0.001, respectively) (Fig. 2 A). Before surgery, deficiencies of iron, ferritin, folic acid, vitamin B12, magnesium, and phosphorus were 22%, 5.3%, 1.4%, 3.8%, 29.7%, and 5.3%, respectively (Fig. 2 B–G). One year post-surgery, deficiencies of ferritin, magnesium, and vitamin B12 worsened (21.3%, 11.7%, and 15.2%; p < 0.001, p < 0.001, and p = 0.001, respectively), whereas there was no significant difference in deficiencies of iron, folic acid, and phosphorus (16.2%, 2%, and 8.1%; p = 0.141, p = 0.645, and p = 0.248, respectively). In addition, 26 (43.3%) out of 60 patients who had a follow-up 6 years after surgery had iron deficiency, while 7 (11.7%) of them had low level of vitamin B12. Deficiencies of ferritin and vitamin B12 was significantly more often seen in patients’ follow-up 6 years post-surgery (p < 0.001 and p = 0.019, respectively). Table 4 shows the number and percentage of micronutrients deficiencies from the 1st to the 6th postoperative year of the follow-up in comparison with the preoperative deficiencies.
Fig. 2
Absolute values and prevalence of nutritional deficiencies before surgery and during the six-year follow-up: (A) haemoglobin, (B) iron, (C) ferritin, (D) folic acid, (E) vitamin B12, (F) magnesium, (G) phosphorus, (H) elevated parathormone (PTH)
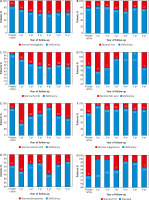
Table 4
Number of deficiencies (percentage in parenthesis) in micronutrients following sleeve gastrectomy
Discussion
Obesity is a current health problem globally and is strongly associated with severe medical conditions as well as elevated mortality rates [11–12]. Bariatric surgery is considered the most effective treatment modality for morbid obesity because nonsurgical management is associated with a lower percentage of WL and poorer improvement in comorbid diseases [11]. Both RYGB and SG are feasible and safe techniques in the clinical setting. However, the American Society for Metabolic and Bariatric Surgery has recently stated that SG is the preferable operation among other BS since 2013 [13].
The first endpoint of this study was to evaluate the evolution of WL after SG as a primary bariatric surgery. The mean % EWL in our study was 80.9%, 79.1%, 73.8%, 71.8%, 71.5%, and 64.9% after 1, 2, 3, 4, 5, and 6 years, respectively. In the AlKhalid et al. study, in which 187 patients were included, the mean % EWL was 58.8%, while Aridi et al. reported a mean % EWL of 69.8% at 5 years [6, 14]. In addition, a systemic review of 16 long-term studies including 492 patients undergoing SG revealed the % EWL to be 62.3% and 53.8% at 5 and 6 years of follow-up, respectively [15]. Our results as well as other data available in the literature illustrate the role of SG in achieving a significant WL over short- and long-term follow-up. Long-term results of SG are scarce because a high rate of patients fail to complete the long-term follow-up [16].
The second endpoint of the current study was to assess the prevalence of preoperative nutritional deficiencies in patients undergoing SG and to evaluate their nutritional status during a 6-year follow-up. According to the literature, nutritional deficiencies are a common long-term clinical problem in patients undergoing bariatric procedure because of modifications caused to the gastrointestinal physiology, which could lead to macro- and micronutrient absorption [17]. However, SG is a non-malabsorptive bariatric surgery with a lower risk of developing postoperative nutritional deficiencies [9]. Patients undergoing RYGB, which is a malabsorptive bariatric procedure, are at higher risk of developing these postoperative deficiencies [18]. By resecting the gastric fundus during SG, a considerable reduction in the number of parietal cells occurs, and less intrinsic factor is produced, leading to an impaired absorption of several micronutrients such as vitamin B12 and iron [9–10]. Postoperative nausea and vomiting, reduced food intake, and subsequent poor food choices are some other reasons why patients undergoing SG might have nutritional deficiencies [19].
Our study shows that micronutrient deficiencies are frequently found pre-operatively in obese patients. Although there are many reports showing the importance of pre- and post-operative evaluation of the nutritional status of patients undergoing a bariatric surgery, few of them reported on long-term micronutrient deficiencies following SG [18, 20, 21]. The presence of nutritional deficiencies preoperatively has been shown to be predictive of postoperative deficiencies [22].
Anaemia can be caused by micronutrient deficiencies and is determined based on low haemoglobin levels. According to the results of our study, anaemia was found in 17.2% of patients preoperatively. Other studies found preoperative anaemia rates between 12% and 22% [21, 23, 24]. The number of anaemic patients in our study increased in the 1st (27.4%) as well as in the 6th (36.7%) postoperative year. Postoperative anaemia after bariatric surgery is in most cases due to iron deficiency, along with vitamin B12 deficiency as a secondary cause [17]. Preoperatively, 22% of our patients had iron deficiency and 5.3% had low serum ferritin. The prevalence of these deficiencies was increased in the 6th postoperative year (25%, and 43.3%, respectively). Other studies describe iron deficiency rates between 17% and 44% [21, 25]. Serum ferritin level is a more specific marker than serum iron levels for detecting iron deficiency [26]. Alexandrou et al. reported that iron deficiency, expressed by low levels of serum ferritin, occurs in more than 30% of patients 5 years post-surgery, having a similar rate after SG and RYGB [27]. Folic acid deficiency was already present in 1.4% of the patients of our study preoperatively. The prevalence of this deficiency remained low (0–2%) in the 6-year follow-up period. The deficiency of folic acid is mostly associated with reduced intake of food rich in folic acid [28]. This deficit is a potential complication of bariatric operations that can lead to anaemia. The prevalence of folic acid deficiency after both malabsorptive and restrictive surgeries ranges from 9 to 39% [17, 29]. Postoperatively, 3.8% of our patients had low levels of vitamin B12 (cobalamin). The prevalence of vitamin B12 deficiency was significantly higher in the 1st postoperative year (15.2%) as well as in the 6th postoperative year (11.7%). Damms-Machado et al. also reported a worsening of vitamin B12 levels after SG [30]. This deficit is a major cause of anaemia in patients undergoing RYGB, with a prevalence of 19–35% after 5 years [31]. Cobalamin deficiency is less common in patients undergoing SG, with a prevalence of 3–18%, supporting our findings [10, 20]. Supplementation of vitamin B12 is recommended after malabsorptive procedures such as RYGB, to avoid neurological and psychiatric symptoms caused by a lack of this vitamin. However, there is no evidence of benefits after restrictive procedures [17]. Prior to surgery deficient serum levels of magnesium were diagnosed in 29.7% of patients. Postoperatively, the magnesium deficiency rates were found to be between 11.7 and 21.4%. This is higher than the prevalence reported in other studies. In the van Rutte et al. study, which included 407 patients, magnesium deficiency was found in 2% of patients preoperatively and 3% in the 1st year postoperatively [10]. The deficit of this mineral is associated with neurological and cardiovascular symptoms [32]. Low levels of serum phosphorus were noticed in 5.3% of our patients before undergoing SG. Postoperatively, the prevalence of phosphorus deficiency was between 3 and 9.7%. Van Rutte et al. found hypophosphataemia in 14% of patients preoperatively, whereas in the first postoperative year 3.5% of patients were diagnosed with phosphorus deficiency [10]. Prior to surgery 2.9% of the patients of the current study had raised PTH levels (Fig. 2 H), rates of hyperparathyroidism decreased over 6 years of postoperative follow-up, but there were no significant differences between pre- and postoperative prevalence of hyperparathyroidism. Ben-Porat et al. found elevated PTH levels in 40.9% of patients preoperatively, which improved 1-year post-surgery (10%) [9]. The presence of elevated levels of PTH before bariatric surgery is common in obese patients, suggesting imbalance in the calcium-vitamin D axis [33]. Vitamin D and parathormone are 2 major regulators of bone metabolism. Parathormone is an essential stimulator of vitamin D synthesis in the kidney, while vitamin D can cause negative feedback on parathormone secretion. Vitamin D and parathormone play crucial roles in maintaining the balance of phosphate and calcium. The former can elevate levels of calcium as well as cause suppression of phosphate metabolism [34].
Our study has several limitations because patients were assessed retrospectively from a single centre. In addition, we did not examine levels of other vitamins and minerals such as calcium, vitamin D, and zinc. Furthermore, we only recommended patients to take supplements for preventing nutritional deficiencies or treat them without examining the compliance to our recommendations, and we did not record the actual consumption of the supplements recommended. Another limitation of this study is the fact that we examined the appearance of nutritional deficiencies after SG in a single sample including both males and females, without comparing each deficiency between 2 sexes. The comparisons were made between pre- and postoperative results of patients’ nutritional status. The lack of assessment of the diet is another limitation of our study.
Conclusions
In conclusion, this retrospective single-centre study demonstrates that SG is an effective type of bariatric surgery that achieves sustained long-term weight loss. Furthermore, it is worth mentioning that a significant number of patients with obesity present nutritional deficiencies prior to surgery, the most important being magnesium and iron deficiencies. The outcomes of this study show the important role of evaluation and assessment of nutritional deficiencies in obese patients before and after a bariatric procedure such as SG. Knowledge of micronutrient deficiencies in these patients is beneficial for both prevention and management of nutritional complications associated with SG with the administration of oral nutritional supplementation according to the patient’s needs. In addition, control of the diet and dietary care are necessary to maintain a proper nutritional status and avoid nutritional deficiencies.











