Introduction
Type 2 diabetes mellitus (T2DM), which is the more prevalent form of diabetes among the two types, is widely acknowledged as a global pandemic [1]. Diabetes is classified as:
insulin-dependent (type 1) diabetes mellitus (T1DM),
T2DM – a chronic medical condition characterized by high blood sugar levels resulting from the body’s inability to effectively use insulin,
gestational diabetes, – maturity onset diabetes of the young (MODY)
a kind of diabetes that occurs in young individuals and is characterized by a genetic cause,
neonatal diabetes
diabetes that is diagnosed in the first six months of life,
Wolfram Syndrome
a rare genetic disorder characterized by the development of diabetes and other symptoms,
Alström Syndrome,
Latent Autoimmune Diabetes in Adults (LADA),
3c diabetes,
diabetes caused by the use of steroids,
cystic fibrosis-associated diabetes.
It is worth noting that most individuals diagnosed with diabetes, namely over 90% of patients, are affected by T2DM [2]. T2DM poses various costs for governments, encompassing direct, indirect, and intangible aspects. Direct medical costs encompass the expenses related to medical treatments and personnel required for patient care. Indirect costs result from reduced workforce participation in the economically active population. There are also correlations due to the increase in expenses associated with health insurance in addition to medical appointments. These factors lead to behavioral changes in patients, which leads to a decrease in productivity [3]. Hyperglycemia signifies the elevated levels of glucose in the body following glucose intake. Isolated hyperglycemia among individuals with T2DM refers to those who have normal fasting plasma glucose levels and do not exhibit ketoacidosis. Identifying glucose irregularities early and averting the progression from prediabetes to full-fledged diabetes can hold significant importance, as the duration of elevated blood sugar levels might be indicative of potential negative consequences [4].
Among the clinical manifestations observed in individuals with T2DM, patients may exhibit:
high levels of glucose in the blood (hyperglycaemia) [5] associated with symptoms such as heightened thirst, frequent urination, and blurred vision, and weight loss;
random blood glucose measurements exceeding 200 mg/dL (11.1 mmol/L);
insufficient production of insulin resulting from malfunction of pancreatic beta cells that has been identified as a contributing factor [6];
an increased occurrence of gluconeogenesis and glycogenolysis;
dehydration [7-9], accompanied by symptoms such as excessive urination (polyuria), excessive thirst (polydipsia), and osmotic diuresis [10];
the phenomenon of compensatory thirst can result in weight loss and the development of cravings for sugary foods;
the phenomenon of calorie loss resulting from glycosuria and poor glucose use;
symptoms characterized by muscular soreness and abdominal discomfort [11];
lactic acid buildup;
electrolyte and acid-base disturbances, as well as hypokalemia;
a metabolic state of alkalosis and/or acidosis that are physiological disturbances characterized by alterations in the acid-base balance of the organism [12];
imbalances in electrolytes;
the process of ketogenesis involves the synthesis of ketone bodies [13-14].
All the above symptoms occur in the case of T2DM. Symptoms of hyperglycemia typically do not manifest until blood glucose levels above 180 to 200 mg/dL, or 10 to 11.1 mmol/L. Common signs of diabetes include excessive thirst, increased frequency of urination, and persistent fatigue. The symptoms manifest because to the retention of glucose in the bloodstream, where it remains unused as a source of energy. The body attempts to eliminate the surplus glucose through urine. The onset of symptoms of T2DM often occurs over a span of several years. Certain individuals may be asymptomatic. Onset of T2DM typically occurs throughout adulthood, although there is a growing incidence in children and adolescents. Given the difficulty in identifying symptoms, it is crucial to be aware of the risk factors associated with T2DM [14].
There are several screening examinations available for diabetes, examples include the measurement of glycosylated hemoglobin (A1C), assessment of fasting plasma glucose, or undergoing a two-hour oral glucose tolerance test (OGTT) [15]. The A1C test is utilized to diagnose diabetes mellitus in people presenting with the characteristic symptoms of hyperglycemia [16]. Both assessments are capable of efficiently detecting cases of diabetes in individuals who do not exhibit any symptoms. However, there is an ongoing debate regarding the best appropriate screening test [17]. Furthermore, it should be noted that certain persons diagnosed with T2DM may exhibit signs of hyperglycemia, characterized by elevated blood glucose levels surpassing the threshold of ≥200 mg/dL [18].
Hyperglycemia refers to elevated blood sugar levels resulting from insufficient insulin. Isolated hyperglycemia, characterized by a blood sugar level exceeding 200 mg/dl, is typically identified during routine tests or capillary blood glucose monitoring. Importantly, it does not coincide with other metabolic issues such as acidosis or hyperosmolarity [19]. While a recent examination has outlined the advantages and drawbacks of intensive glycemic control in individuals with T2DM [20], there is limited information available regarding control and treatment strategies specifically for isolated hyperglycemia. This review aims to offer a comprehensive and methodical overview of the existing knowledge on T2DM and early warning signs in patients who do not have prediabetes but require management for isolated hyperglycemia.
Aim of the work
The main aims of management T2DM are to maintain blood sugar levels within the desired range, address comorbidities such as hypertension, prevent acute decompensation, delay the onset of long-term complications, reduce mortality, and ensure a high quality of life. The primary objective of diabetes treatment is to restore blood sugar levels to a normal or non-diabetic range. However, achieving this aim can be challenging due to the risk of experiencing frequent episodes of low blood sugar or hypoglycemia.
Effective management of diabetes necessitates a treatment approach that is rooted in a comprehensive understanding of its underlying pathophysiology. Insulin is crucial for managing T1DM as it addresses the issue of impaired insulin secretion. Nevertheless, managing type 2 diabetic patients is more intricate due to the presence of a deficiency in both insulin secretion and insulin action. Hence, the choice of treatment will be contingent upon both the disease’s stage and the specific attributes of the patient. The aim of this article was to analyze the overarching objectives of the treatment and evaluate the strategies for managing T2DM.
Methods
A comprehensive search of scientific literature was conducted and relevant data was extracted from the PubMed, Medline, and EMBASE databases. A total of 479 publications were identified, out of which 421 were excluded due to not meeting the inclusion criteria. The inclusion criteria establish a standardized, dependable, consistent, and unbiased method for identifying the study population. The exclusion criteria encompass conditions or traits that render the recruited group ineligible for participation in the study. These characteristics could potentially operate as confounding variables for the outcome measure, which in turn optimizes the study’s external and internal validity, improves its feasibility, lowers its costs, and minimizes ethical concerns. A total of 42 publications were excluded because they were not relevant to diabetes management. A thorough review was conducted on the remaining 16 papers.
Literature review results
Epidemiology
T2DM is more prevalent among the elderly compared to any other age group. Nevertheless, its occurrence is on the rise among young adults and children because of the growing prevalence of obesity, sedentary habits, and insufficient dietary choices [21].
T2DM, also called (non-insulin dependent), is the most common type of diabetes and is responsible for about 90% to 95% of diabetes cases around the world [22].
The presence of various complications associated with diabetes, such as cardiovascular disease and stroke, plays a major role in the overall mortality rates observed worldwide. The prevalence of T2DM is gradually increasing, accounting for most diabetes cases [23].
Based on a comprehensive analysis of data from 220 countries and territories, as presented in the recently released World Diabetes Atlas of the International Diabetes Federation, it was observed that a significant proportion of the world’s adult population, specifically 8.8% of individuals aged 20 to 79 years, approximately 537 million people have diabetes [22].
According to the latest data provided by the International Diabetes Federation, the current prevalence of diabetes among the age group from 20 to 79 years is 537 million cases. The projected figure indicates that the number of cases is expected to rise to 783 million cases by 2045, especially in middle-income countries where a significant rise in diabetes cases is expected (Table 1). Estimates and statistics on diabetes cases are determined based on the classification provided by the World Bank [24].
Table 1
Statistics of the prevalence of diabetes in the world for the years 2019, 2030, and 2045 [22]
The global population aged 60 to 79 years is affected by diabetes at a rate of 18.6%, and this age group is expected to see the greatest rise in the prevalence of the disease. However, when looking at absolute numbers, the most prevalent age group is individuals aged 40 to 59 years. Diabetes rates are expected to increase at a higher rate due to the higher incidence of diabetes among low-income people. The prevalence of diabetes shows an inverse relationship [22].
Similarly, a negative correlation exists between a nation’s gross domestic product (GDP) and the incidence of diabetes. In other words, countries with lower economic levels tend to exhibit higher rates of diabetes [25,26].
According to data provided by the American Diabetes Association, a significant proportion of patients diagnosed with diabetes, ranging from 33% to 49%, face challenges in effectively controlling their blood glucose and cholesterol levels. This difficulty in managing these health markers is a contributing factor to the increasing prevalence of diabetes worldwide [22]. Various factors, including the presence of various concurrent health disorders, social and financial limitations, and unique obstacles in attaining targeted goals in diabetes therapy, exert a substantial influence [27].
According to conservative research estimates, the anticipated global expenditures for addressing and averting complications associated with diabetes are forecast to amount to $673 billion. However, in a more hopeful scenario, this figure is expected to increase to $1,197 billion. According to leading authorities in the field of epidemiology and disease, it is projected that by the year 2040, the associated expenses are expected to significantly increase to almost $1,425 billion [26].
Physiopathology
Insulin serves as the chief controller of blood sugar levels in humans. The production of insulin is attributed to the beta cells found in the pancreatic islets. Initially, these cells generate proinsulin, which subsequently undergoes processing to form proinsulin. This proinsulin, in turn, undergoes additional decomposition into insulin and C-peptide [28].
When the concentration of glucose surpasses 70 mg/dl, the transportation of glucose into the pancreatic beta cells occurs through the GLUT transporters. Glucose is subjected to enzymatic digestion by glucokinase within the pancreatic islets, resulting in the production of pyruvate and ATP. Consequently, the activation of potassium channels that are responsive to stimuli leads to the subsequent initiation of insulin secretion [29,30].
In T2DM, there is a combination of two factors at play: insulin resistance (IR) and a decline in insulin secretion, both of which promote high blood sugar levels (hyperglycemia). However, comprehending the underlying pathology is intricate because clinical assessments do not typically measure IR and deterioration [31].
The prevailing belief is that the principal catalyst for IR is a lifestyle characterized by physical inactivity and the resultant obesity, while hereditary factors and the aging process are considered to have relatively minor contributions to its onset. In contrast, the primary mechanisms contributing to the decrease in insulin secretion are hereditary influences and the functioning of beta cells in the intrauterine environment. The reason behind reduced pancreatic beta cell function can be attributed to hyperglycemia [32].
T2DM is commonly characterized by alterations in the metabolism of lipids, proteins, and carbs, hence increasing the susceptibility to cardiovascular complications. The development of metabolic alterations may be significantly influenced by hyperinsulinemia that arises because of IR. Moreover, there is a substantial correlation between elevated levels of free fatty acids, oxidative stress factors, and inflammatory cytokines and the emergence of both T2DM and cardiovascular complications [33,34].
T2DM is associated with two separate risk factors, namely reduced insulin production and IR. Furthermore, the progression from glucose tolerance to glucose intolerance is characterized by comparable reductions in glucose disposal, both in terms of insulin responsiveness and glucose-stimulated secretion [35].
The entry of glucose into the cell is facilitated by the utilization of glucosamine (GlcN)-activated sugar, and this mechanism is mediated by the GLUT-2 transporter. The GLUT2 transporter protein is expressed in pancreatic beta cells, as well as in renal and hepatic cells [36]. Research has demonstrated that a high-fat diet can impair this transporter, resulting in glucose intolerance [37,38].
It’s worth noting that a high-fat diet harms beta cells by creating reactive oxygen species within them, and unlike other cells, beta cells lack their own natural antioxidants.
Although a fasting blood glucose level of 126 mg/dl or above is a diagnostic criterion for T2DM, beta-cell activity is affected even when glucose levels reach 100 mg/dl. [39].
IR cannot be considered an independent diagnostic criterion for T2DM as its development in most individuals is attributed to a hereditary predisposition towards T2DM. This phenomenon is apparent as IR tends to exacerbate with higher body weight and older age, suggesting a malfunction in beta-cell function among persons who are at a higher risk of developing impaired glucose tolerance. In instances where individuals exhibit a normal body weight but possess an elevated susceptibility to the development of T2DM, the presence of post-glucose and fasting hyperinsulinemia renders them more prone to the accumulation of body weight, thus resulting in the occurrence of hyperglycemia. The phenomenon described can potentially exacerbate the deterioration of beta-cell function as a result of glucotoxicity, leading to a decrease in the level of expression of the gene that codes for insulin [40].
The typical degradation of proinsulin leads to the routine synthesis of insulin, constituting around 10-15% of the overall insulin secretion. Nevertheless, in the context of T2DM, the pancreatic beta cells exhibit an inability to adequately produce or metabolize the necessary levels of insulin that are essential for proper bodily function. On the other hand, T2DM is distinguished by an elevation in proinsulin synthesis during periods of inactivity [41]. When the continued secretion of greater levels of proinsulin is observed, even after adjusting for the level of obesity, it indicates a dysfunction of the beta cells, rather than just an increased demand caused by IR associated with obesity. Furthermore, it indicates that the beta cells’ ability to transform proinsulin into insulin is compromised [42,43].
Clinical presentation
In general, individuals who are afflicted with solitary hyperglycemia tend to lack the characteristic manifestations commonly associated with elevated blood glucose levels, including heightened thirst (polydipsia), frequent urine (polyuria), excessive appetite (polyphagia), and weight reduction. In addition, it is typically seen that severe manifestations such as ketoacidosis, characterized by symptoms such as hyperventilation, asthenia, nausea, vomiting, and a discernible ketone odor, are frequently not present. Alternatively, this condition is likely to present itself as either diabetic ketoacidosis or non-ketotic hyperosmolar hyperglycemic decompensation [44].
Diagnosis
After completing the initial diagnostic steps to identify isolated hyperglycemia and exclude ketosis, it is advisable to conduct supplementary tests. If any level of ketosis is detected during these tests, further investigations for ketoacidosis should be pursued, although this aspect is not covered in this section. It is imperative to acknowledge that the practice of diagnosing T2DM using urine glucose measurement is discouraged owing to its restricted sensitivity [45].
Management
The treatment approach for T2DM should encompass a comprehensive strategy, which involves the use of hypoglycemic medications, adopting lifestyle modifications, engaging in physical activity, and following a dietary plan that aims to either maintain a normal weight or achieve a BMI below 25 [46]. The use of efficient strategies to manage hyperglycemia has the potential to play a significant role in the prevention of the advancement of microvascular problems, including retinopathy, nephropathy, and neuropathy [47].
The initial guidelines for patients diagnosed with T2DM who have a body mass index (BMI) falling between the range of 25 to 29.9 kg/m2 (indicating overweight) or surpassing 30 kg/m2 (indicating obesity) consist of two primary components: weight loss and engaging in moderate physical activity. It is recommended that these individuals strive to achieve a decrease in their initial body weight by 5-10% and endeavor to participate in a minimum of 30 minutes of moderate physical activity on a regular basis throughout the week [48].
Initiation of treatment is recommended when blood glucose levels above 250 mg/dl or when ketosis is detected. The preferred initial treatment involves the subcutaneous delivery of ultra-fast acting insulins. In instances of heightened hyperglycemia, particularly when glucose levels surpass 400 mg/dl or when ketosis is identified, it is recommended to administer rapid-acting intravenous insulin. The suggested dosage entails diluting 100 IU in 100 ml of saline solution and administering it by infusion at a rate of 0.1 IU per kilogram per hour [49]. Furthermore, if there are no specific counterindications, using metformin is recommended as the first treatment for patients who have recently been diagnosed with T2DM and do not show any symptoms. The initial dosage is 500 mg per day, taken with the evening meal. If well-tolerated, a further 500 mg dose is administered with breakfast. Upon receiving a recent diagnosis of T2DM, most individuals will promptly commence treatment with a medication known as metformin (often sold under brand names such as Glucophage, Glumetza, Riomet, and Fortamet). Metformin enhances insulin sensitivity to decrease elevated blood glucose levels [50].
The determination of the appropriate insulin dosage required to rectify the present blood glucose level is contingent upon many aspects, encompassing the patient’s specific blood glucose measurement, the intended goal blood glucose level (established at 170 mg/dL), and the individual’s Insulin Sensitivity Factor (ISF). ISF is a measure of the rate at which blood glucose levels decline in response to the administration of rapid-acting insulin. It is determined by the equation ISF = 1800/t, where “t” indicates the total dose of insulin provided to the patient [51].
In cases where blood glucose levels exceed 400 mg/dl and the patient experiences dehydration and ketosis, fluid replacement is essential. Crystalloid solutions are administered if the patient can tolerate this route, while oral rehydration salts, water, or lemon juice are preferred alternatives.
In the case of patients exhibiting blood glucose levels over 400 mg/dl, it is imperative to undertake a reassessment and administer an additional measurement of capillary blood glucose. If the utilization of ultra-rapid insulin is implemented, it is recommended that the reassessment be conducted within a timeframe of 2-3 hours. In contrast, if the administration of rapid-acting human insulin takes place, it is advisable to undertake the evaluation within a time frame of 3-4 hours [52].
If the blood glucose level remains above 200 mg/dl during the reassessment, a second dose will be administered, considering the blood glucose value obtained during this reassessment [53].
In general, for patients dealing with isolated hyperglycemia, it is advisable to follow these recommendations:
Pharmacological management
The reduction in hemoglobin A1c levels resulting from the use of the U.S. Food and Drug Administration (FDA) approved medicines for the management of hyperglycemia in individuals with T2DM generally falls within the range of 0.6% to 1.5%. While there may be some discrepancies among the guidelines published by different medical associations, they do agree on several fundamental principles. These principles include: setting a specific target for glycemia or hemoglobin A1c levels [54], initiating treatment with metformin for most patients [55]. Metformin is a longstanding drug for treating T2DM, widely utilized for its antidiabetic properties. The method of action involves inhibiting hepatic glucose synthesis, reducing insulin resistance, and enhancing insulin sensitivity. The medicine has undergone thorough investigation and has demonstrated efficacy in reducing blood glucose levels without elevating the risk of hypoglycemia. It has been employed for the management of obesity, gestational diabetes, and polycystic ovary syndrome. Metformin is recommended as the initial treatment option for diabetes, as per the current guidelines, in light of the current American Diabetes Association/European Association for the Study of Diabetes and PTD recommendations. Metformin has traditionally been recommended as the initial treatment for T2DM due to its high effectiveness in reducing HbA1c levels, low risk of hypoglycemia when used alone, ability to maintain weight or even cause slight weight loss, favorable safety profile, and affordable cost. Nevertheless, there is a current recognition that alternative methods may be suitable. Importantly, the positive effects of GLP-1 RA and SGLT2i on cardiovascular and renal outcomes are not influenced by the use of metformin. Therefore, these medications should be considered for those with existing or high risk of cardiovascular disease (CVD), heart failure (HF), or chronic kidney disease (CKD), regardless of whether they are using metformin or not [56,57]. At the beginning of treatment, it may be advisable to try early combination therapy if there is a perceived need for better control of blood sugar levels or to protect the heart and kidneys. This can help prolong the period until treatment becomes ineffective [58]. Metformin is contraindicated in individuals with an estimated glomerular filtration rate (eGFR) below 30 ml/min per 1.73 m2, and dosage adjustment should be contemplated when the eGFR falls below 45 ml/min per 1.73 m2 [59]. Metformin usage can lead to decreased levels of vitamin B12 in the bloodstream and exacerbation of neuropathy symptoms. As a result, it is typically advised to regularly evaluate and supplement vitamin B12 levels if they are insufficient, especially in those with anemia or neuropathy [60,61], using combination therapy to achieve glycemic targets [62], preventing episodes of hypoglycemia [63], and understanding the adverse effects associated with medications (Figure 1) [64]. The major choice of metformin as a therapy option is justified by its efficacy in reducing blood glucose levels, its established safety profile, its weight-neutral characteristics, and its cost-effectiveness. It is recommended to exercise caution in the titration of metformin medication to mitigate potential gastrointestinal adverse effects. In addition, there is a scarcity of evidence derived from a cardiovascular outcomes study of restricted scale, as well as cohort data, which suggest the existence of potential cardioprotective advantages linked to the use of metformin.
Figure 1
Glucose management for patients with type 2 diabetes
Source: Own elaboration based on Bays et al. [65].
Combination therapy
According to the guidelines provided by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD), it is recommended to combine metformin with any secondary drug that has received approval from the FDA [66]. On the other hand, the American Association of Clinical Endocrinologists supports the utilization of either incretin-based therapy or sodium glucose transporter 2 (SGLT2) inhibitors [67]. The primary method for treating T2DM globally is through the use of incretin-based medicines, which involve the use of GLP-1 mimics and DPP-4 inhibitors. The distinctive mechanism of incretin-related pharmaceuticals can be highlighted by comparing them with existing glucose-lowering medications. Incretin preparations that modulate both insulin and glucagon secretion in a way depending on blood glucose levels exhibit a little risk of hypoglycemia and weight gain. DPP-4 inhibitors function by suppressing the activity of the enzyme responsible for degrading incretin hormones, hence preserving the natural level of circulating GLP-1 at approximately 10 pmol/L. GLP-1 receptor agonists, in contrast, exert their effects at concentrations higher than what is naturally present in the body. This leads to a stronger impact on controlling blood sugar levels and causes weight reduction by slowing down the emptying of the stomach and increasing the feeling of fullness in the brain. According to reports, incretin-related medications demonstrate greater efficacy in Asian patients with T2DM compared to other ethnic groups [68]. Specifically, the DPP-4 inhibitor is found to be more beneficial in individuals with a lower body mass index, while the GLP-1 receptor agonist is more effective in those with a body mass index below 30 kg/m2. Regardless of its pharmacokinetics, each DPP-4 inhibitor exhibits a comparable ability to reduce glucose levels. When administered over an extended period, it is believed that the HbA1c reducing effectiveness can range from 0.5% to 1.0%. Furthermore, a robust system for ensuring the safety of older individuals in administration has been implemented. DPP-4 inhibitors can be used safely and efficiently [69], even in cases of end-stage renal failure, by appropriately reducing the dosage. However, it is important to note that linagliptin and teneligliptin can be used without any dose reduction because they are cleared from the body by non-renal pathways. Currently, there are two DPP-4 inhibitors, namely trelagliptin and omarigliptin, which are primarily accessible in Japan and various Asian nations [70]. Understanding the specific clinical characteristics of patients who are susceptible to side-effects is of great significance. Age and renal function had a positive correlation with gastrointestinal adverse events. A recent investigation indicated that individuals who are already using proton pump inhibitors or histamine-2 receptor antagonists had a higher likelihood of experiencing gastrointestinal problems after the administration of GLP-1 receptor agonists. However, the exact mechanism behind this phenomenon remains unclear. After 15 years of clinical use, it has been discovered that incretin-related medications have diverse effects beyond their ability to reduce glucose levels (Figure1). GLP-1 receptor agonists have a notable advantage in terms of their ability to prevent cardiovascular problems. Regrettably, there is currently a lack of dependable evidence that definitively confirms the anti-arteriosclerotic properties of DPP-4 inhibitors [71]. The cardiovascular outcome studies have yielded valuable insights that have influenced modifications in the ADA/EASD consensus guideline for the treatment of type 2 diabetes. Simultaneously, the processes underlying the cardiovascular benefits are also under investigation [72]. It is more evident that GLP-1 receptors are present in vascular endothelial cells, as indicated by recent research. The anti-arteriosclerosis activity of GLP-1 is attributed to both its direct impact on vascular endothelial cells through GLP-1 receptors and its indirect effects associated with the enhancement of metabolic profile [73]. If a patient cannot tolerate or has a medical reason to avoid using metformin, alternative treatment options include sodium-glucose cotransporter 2 (SGLT-2) inhibitors, sulfonylureas, incretin-based therapy (DPP-4 inhibitors and GLP-1 receptor agonists), and peroxisome proliferator activated receptor gamma (PPAR-γ) agonist (pioglitazone). In cases where the patient is obese or at a high risk of hypoglycemia, it is recommended to use incretins and SGLT-2 inhibitors. However, individuals with heart failure should not use PPAR-γ agonists [74]. It is imperative to acknowledge that practitioners ought not to postpone the introduction of a second drug until the patient’s glycemic control deteriorates [75].
Historically, the management of inadequate insulin secretion involved the utilization of either insulin secretagogues, such as glibenclamide or glipizide, or insulin replacement therapy [76]. Both alternatives have exhibited efficacious glucose-lowering properties; nevertheless, they are accompanied by the potential hazards of hypoglycemia and weight gain. Pioglitazone, an alternative efficacious option, does not feature among the drugs endorsed by the American Association of Clinical Endocrinologists. The exclusion of this medication may be attributed to concerns surrounding an increased likelihood of heart failure and weight gain linked to its usage [77].
Effective use of insulin
In cases where a patient’s hemoglobin A1c level surpasses 9% (with a desired aim of below 7%) and they are now being treated with metformin and non-insulin medicines, the implementation of insulin therapy becomes imperative. The process of modifying basal insulin dosage to get desired fasting blood glucose levels can be appropriately and efficiently managed by either the patient or their healthcare provider. A frequent approach entails commencing therapy with a conservative dosage of extended-release insulin before sleep, often at approximately 10 units, and afterwards making incremental modifications to achieve a fasting blood glucose concentration below 120 mg/dL. The initial dose is unlikely to cause hypoglycemia; however, it is crucial to adjust the insulin dosage, typically necessitating a range of 30 to 50 units [78].
Criteria for commencing insulin therapy in T2DM:
recently diagnosed diabetes (with the possibility of reverting to the standard treatment plan and discontinuing insulin therapy) [74];
blood glucose levels equal to or greater than 300 mg/dL (16.7 mmol/L) accompanied by clinical symptoms of high blood sugar
ineffectiveness of treatment without the use of insulin (HbA1c levels above target despite intensive behavioral therapy);
algorithm for insulin management in type 2 diabetes [74].
When experiencing high blood sugar levels in the morning, using long-acting analogues can decrease the chances of experiencing low blood sugar levels during the night and severe episodes of low blood sugar. If high blood sugar levels are present during fasting and throughout the day, it is recommended to administer short or rapid-acting insulin injections in the morning. This is especially important if high blood sugar levels persist after meals [74].
If the daily basal insulin requirement exceeds 0.3-0.5 units per kilogram of body weight and blood sugar levels are not well controlled, it may be appropriate to consider intensifying treatment. This can be done by using mixed insulin, biphasic insulin analogue, or premixed insulin analogues. Another option is to supplement long-acting insulin (administered once or twice daily) with a short-acting insulin or rapid-acting insulin analogue, which is administered at 1-3 meals. This approach is known as a basal-plus insulin regimen or intensive insulin therapy. One should contemplate ceasing the use of insulin secretagogues, when administering daily insulin doses greater than 100 units, which suggests the presence of insulin resistance, it is important to investigate the underlying causes and be mindful of potential negative consequences. It is recommended to try to decrease insulin resistance by administering a continuous infusion of insulin, either subcutaneously or intravenously, for a period of 72 to 96 hours [74].
The integration of basal insulin into any therapeutic plan is feasible. The combination of basal insulin plus metformin, GLP1RA (glucagon-like peptide-1 receptor agonists), SGLT2 inhibitors, or pioglitazone has been demonstrated to be a safe and effective approach for achieving optimal glucose control. In recent times, the FDA has granted approval for two variations of single-injection therapy that combine long-acting insulin with GLP1RA. The combination has exhibited notable efficacy in reducing glucose levels, while also being associated with weight neutrality or weight reduction, and a low incidence of hypoglycemia. To optimize compliance and boost overall results, it is imperative to take into account the effectiveness, potential bad reactions, and financial implications associated with each pharmaceutical intervention [79].
The treatment of T2DM
Phases of treatment for T2DM according to the Polish Diabetology Association [74]:
Commencement of treatment:
Lifestyle adjustment involves reducing the number of calories in meals and increasing physical activity for at least 30-45 minutes per day to decrease body weight.
Pharmacological treatment can be started with either a single medication or a combination of medications. Metformin, SGLT-2 inhibitors, and GLP-1 receptor agonists are recommended as the initial medications for treating T2DM.
GLP-1 receptor agonists and SGLT-2 inhibitors, which have demonstrated benefits, should be prioritized for individuals with cardiovascular disease, multiple risk factors, or chronic kidney disease. Individuals with heart failure should avoid using the PPAR-γ agonist and saxagliptin. The effectiveness of the oral treatment can only be evaluated after several weeks of use. When deciding to start combination therapy for newly diagnosed diabetes, it is important to consider the presence of atherosclerotic cardiovascular disease, heart failure, chronic kidney disease, multiple cardiovascular risk factors, or severe hyperglycemia (HbA1c > 8.5%). Patients belonging to the high-risk groups should incorporate an SGLT-2 inhibitor and/or a GLP-1 receptor agonist into their combination treatment plan [74].
Escalation of treatment through the administration of oral medicines or GLP-1 receptor agonists:
Lifestyle modification and the addition of a medicine from a previously unused class, such as metformin, SGLT-2 inhibitor, incretin medication (DPP-4 inhibitor or GLP-1 receptor agonist), sulfonylurea, or PPAR-agonist gamma, either as monotherapy or in combination therapy. When selecting medication, it is important to consider comorbidities, such as confirmed cardiovascular disease and chronic kidney disease, as well as coexisting obesity, risk of hyperglycemia, and the patient’s financial resources. This diabetes consideration should be made early in the treatment process. Patients with atherosclerotic cardiovascular disease, heart failure, chronic renal disease, or numerous cardiovascular risk factors should prioritize the use of drugs that have been proved to have favorable effects on the progression of these disorders, as well as on the rates of total and cardiovascular mortality. The efficacy of several SGLT2 inhibitors has been scientifically shown. Other drugs also activate the GLP-1 receptor [80].
Lifestyle changes and a treatment plan consisting of either three or four drugs, including metformin as a constant, along with two other pharmaceuticals from the following groups: SGLT2 inhibitors, GLP-1 receptor agonists, sulphonylurea.
DPP-4 inhibitors, and PPAR-γ agonists. The selection of drugs at this stage is determined by the same criteria as the previous stage and follows general guidelines for combining antihyperglycemic medications [80,81].
Intensification of insulin therapy:
Lifestyle modifications and straightforward insulin therapy [mainly utilizing basal insulin (NPH insulin, long-acting analogue)]; different approaches while still taking metformin and other oral medications or a GLP-1 receptor agonist through injections, particularly if obesity is present. Patients who are receiving their initial injection therapy, such as basal insulin or a GLP-1 receptor agonist, can achieve intensification by using combination formulations that contain a consistent ratio of basal insulin and a GLP-1 receptor agonist (known as fixed-ratio combination – FRC) [74,80].
Lifestyle changes and combination insulin therapy are recommended to be continued. Administration of metformin and other oral drugs, such as incretin drugs, pioglitazone, flozin, or a GLP-1 receptor agonist via injections, is recommended, particularly in cases of persistent excessive body mass. At each stage of treatment, it is important to attain specific glycemic goals and body weight targets for each individual [74,80].
Streamlining the treatment model for controlling high blood sugar:
Many patients with T2DM require a simplified and less burdensome treatment approach, particularly when it comes to insulin therapy. This may involve relaxing the target blood glucose level for certain patients, such as those at high risk of low blood sugar, patients with cognitive impairments, patients who struggle to follow medical instructions, patients with a limited life expectancy, and patients whose quality of life is negatively affected by a complex treatment regimen [74,80]. One way to achieve this simplification is by reducing the number of insulin injections and doses, using a personalized combination of non-insulin medications to control high blood sugar.
Medical nutrition therapy (MNT) and its importance in diabetes prevention and management
Unhealthy dietary patterns and a sedentary lifestyle have been identified as significant modifiable factors that can mitigate the risk of developing T2DM. The phenomenon of growing urbanization in world, coupled with the broad accessibility of Western-style processed foods that are rich in refined carbohydrates, saturated fats, and added sugars, has brought about substantial changes to the local food environment. The adoption of altered dietary patterns, along with an increase in sedentary lifestyles, has been associated with detrimental consequences on the emergence and advancement of T2DM among the world population. Medical nutrition therapy (MNT) is a methodical technique aimed at optimizing dietary selections to enhance metabolic control and enhance treatment results in individuals with T2DM. The core of MNT entails the provision of counseling and recommendations by a registered dietitian (RD), under the strict supervision of consultant diabetologists [82].
The ADA, AACE, and IDF stress the significance of integrating medical nutritional therapy (MNT) as the primary approach to handle T2DM. They offer uniform guidelines for the daily nutritional needs in T2DM management. MNT goes beyond just limiting calorie intake and controlling portion sizes; it involves a holistic transformation of one’s way of life.
The implementation of MNT on a global scale encounters unique challenges due to the world’s rich cultural diversity and varied culinary traditions, which often feature high levels of sugars and carbohydrates [83].
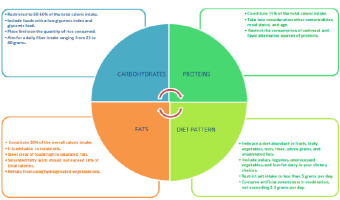
Enforcing nutritional guidelines and advocating for a well-balanced, calorie-controlled diet, alongside regular engagement in physical activity, has been demonstrated to correlate with a decreased occurrence of diabetes. This approach has also resulted in enhancements in several markers, including body mass index (BMI), waist circumference, fasting blood sugar levels, and other relevant factors (Figure 2).
Conclusions
The attainment of patient-centered diabetes management requires the implementation of a comprehensive strategy that encompasses modifications to one’s lifestyle and the utilization of a variety of therapeutic interventions. Metformin is widely recognized as the preferred initial treatment option. Recent pharmaceutical treatments, such as GLP1 (glucagon-like peptide-1) and SGLT2 inhibitors, not only demonstrate efficacy in decreasing glucose levels but also provide the added benefits of weight reduction and potential mitigation of cardiovascular risks. Furthermore, insulin therapy is commonly regarded as a safe and efficacious alternative for those whose glycemic levels remain unregulated despite the utilization of non-insulin interventions.
In the case of younger, medically fit persons, who have just received a diagnosis of diabetes, it is advisable to strive for the maintenance of a hemoglobin A1c level that is below 7%. In the case of elderly individuals with coexisting medical issues, it is imperative to embrace glycemic objectives that are less strict, with an emphasis on ensuring safety and reducing the likelihood of hypoglycemia.
In addition, it is imperative to incorporate evidence-based strategies for managing cholesterol and blood pressure alongside the management of hyperglycemia in order to mitigate the likelihood of cardiovascular problems. Although the cardiovascular advantages associated with SGLT2 inhibitors and GLP1 agents are significant, it is imperative to acknowledge that these pharmaceutical interventions do not serve as substitutes for statin therapy or the control of blood pressure to effectively mitigate the risk of cardiovascular disease.