INTRODUCTION
Retinitis pigmentosa (RP) is a retinal disease involving the death of photoreceptor cells – cones and rods [1]. Vision loss in retinitis pigmentosa results not only from progressive degeneration of photoreceptors but also from the overlap of other macular abnormalities, such as cystoid macular edema (CME), macular hole (MH), epiretinal membrane (ERM), vitreoretinal traction syndrome, as well as glaucoma and cataract. The purpose of this paper is to review the current state of knowledge on macular lesions in retinitis pigmentosa and the current treatment and management options for this condition, and to present our own experiences.
RETINITIS PIGMENTOSA
Retinitis pigmentosa shows autosomal-recessive (in 50-60% of cases), autosomal-dominant (AD) (in 30-40%) or X-linked (in 5-15%) inheritance. RP and other retinal dystrophies are conditions resulting from mutations in more than 250 genes [2]. The incidence of RP worldwide is estimated at 1/4000 births, which adds up to more than a million people affected [1]. Inherited retinal diseases, of which RP and Stargardt’s disease are the most common, are the leading cause of blindness in the working-age population (16-64 years) in England and Wales [3]. Prior to the widespread introduction of optical coherence tomography (OCT) into clinical practice, CME in patients with RP was diagnosed by fluorescein angiography or during a slit-lamp examination. The prevalence of CME among patients with RP was estimated at 10-20% [4]. The frequency of CME diagnosis increased significantly after the introduction of OCT. In 1999, researchers first assessed the prevalence of CME in patients with RP independently of fluorescein angiography and the condition was detected in 13% of patients [5]. In 2008, Hajali’s study estimated the preva- lence of CME on OCT as 38% of patients with RP [6]. In 2019, Liew et al. published the results of a study during which they determined the prevalence of CME as 58.6% of patients [7].
To date, there have been a few studies describing the risk factors for CME in patients with RP, which could be useful in understanding the pathophysiology of and appropriate management and treatment options for this complication [7-9]. The available findings suggest that the occurrence of CME is associated with younger patient age, and is more likely in AD inheritance and least likely in X-linked inheritance [7]. CME is less likely to occur in the presence of ERM or cataract [7, 10]. These findings suggest that the pathophysiologic process leading to CME in RP may differ from that leading to cataract and epiretinal membrane as these latter two conditions do not appear to frequently coexist. There is a hypothesis that a relatively healthy retina is required for CME to occur. This would explain why younger patients and milder forms of RP (such as the AD form) are more prone to CME than more severe forms (X-linked RP). It would also explain the inverse relationship between CME incidence and ERM and cataracts, since the latter two conditions occur at older ages, when there is a smaller amount of healthy retinal tissue [8]. Mutations in the retinal pigment epithelium interfere with the synthesis of certain growth factors. Stress caused by apoptosis in rod cells in the periphery leads to the ectopic synaptogenesis of the central Muller cells. Muller cells undergo compensatory hypertrophy and synthesize excessive growth factors [11]. It seems that not every case of CME should be subjected to treatment attempts because not everyone will get the benefit of treatment [12, 13]. Cystoid macular edema therapy should only be considered if the edema is excessive and long-lasting, which leads to disruption of synaptic connections in the neurosensory retina and increased neurodegeneration and impairs central vision, or if inflammation appears. Arslan, according to his clinical experience, recommends treatment when the thickness of the central macula exceeds 500 μm and patients’ central vision deteriorates [11].
TREATMENT OPTIONS FOR CYSTOID MACULAR EDEMA
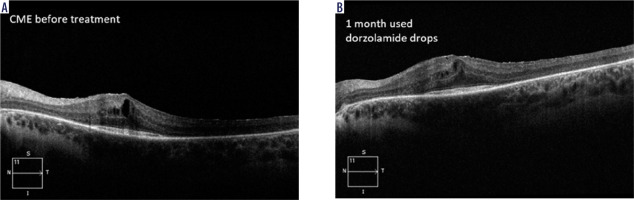
Carbonic anhydrase inhibitors (CAIs) have been used to treat RP-CME for more than 30 years [14-17]. A study showed a significant reduction in central macular thick- ness (CMT) compared to the baseline after treatment with topical CAIs [16]. Topical steroids have also been shown to be useful in treatment of RP-CME. In addition, two studies compared the effect of an intravitreal implant with dexamethasone (0.7 mg, Ozurdex) to topical CAIs, and showed better results in reducing CMT using the implant [18, 19]. Figure 1 shows an OCT image of a patient with CME prior to and after one-month treatment with 2% dorzolamide drops applied twice a day. A reduction in CME and an improvement in VA from 0.1 to 0.2 on the Snellen chart were observed.
Figure 1
HD OCT in a patient with RP-CME prior (A) to and after one-month therapy with 2% dorzolamide drops (B)– own experience
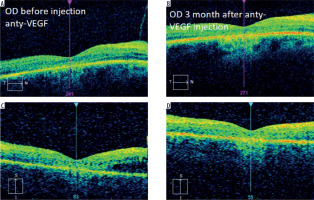
Intravitreal anti-VEGF drugs are another treatment option for CME in patients with RP. The efficacy of these therapies has varied depending on the study and medications used. In 2006, Melo et al. evaluated the effect of bevacizumab (Avastin) in 2 patients with CME associated with RP. In the conclusion of their study, bevacizumab was found to be ineffective in treating this complication [20]. In 2009, Artunay et al. published the results of a study of 7 patients in whom a single dose of ranibizumab had no statistically significant effect on CMT compared to a control group at 6-month follow-up evaluation [21]. In 2016, Strong et al. presented an interesting case of a patient with RP and concomitant CME in whom there was a reduction in CMT after two injections of aflibercept despite a previous minimal response to intravitreal ranibizumab [22]. The same author in 2020 confirmed in his subsequent prospective study that aflibercept may have a favorable impact on CMT in up to 37.9% of patients [14]. Of note, despite demonstration of an anatomic response, no statistically significant improvement in best-corrected visual acuity was observed. These observations are in line with our experience, where CMT reduction was not associated with VA improvement (Figure 2).
Another attempt to treat RP-CME involved employment of a subliminal micropulse laser (SL-MPL), which is used to treat macular disease. The short subliminal pulses prevent thermal damage. No coagulation scars form during SL-MPL treatment. Subliminal induction of retinal pigment epithelial cells leads to the release of some regenerative growth factors and to suppression of inflammatory cytokines, such as vascular endothelial growth factor (VEGF) inhibitors and the pigment epithelium-derived factor (PEDF). According to Arslan et al., SL-MPL appears to be an effective and safe method in non-inflammatory and refractory CME in patients with RP [11]. However, because of the small number of studies, no far-reaching conclusions can be drawn regarding the effectiveness of RP-CME treatment with the micropulse laser.
MACULAR HOLE
Full-thickness macular hole (FTMH) is a rare (0.5-4.5% incidence) but vision-threatening condition in patients with RP. So far, only studies on small groups of patients have been published. According to the available literature, FTMH is commonly treated with vitrectomy with or without ILM (internal limiting membrane) peeling. Closure rates reach 90%, vision improvement has been observed in 62.5% of patients, and failure of hole closure is higher in RP cases with high myopia [23], chronic FTMH [24] and diabetic retinopathy [25]. There are documented cases of FTMH in patients with RP who had posterior staphyloma with significant choroidal and retinal atrophy despite normal eye length (23.24 mm and 23.43 mm) [26]. Surgical treatment of this type of FTMH is difficult due to poor retinal adhesion to the pigment epithelium within the staphyloma. However, closure of the FTMH is important to maintain residual central vision and slow down further photoreceptor degeneration in the long term [27]. The inverted ILM flap technique has been proven effective in patients with associated posterior staphyloma [28]. In the case of resurgery, an ILM flap can be replaced by a lens capsule flap, which was first introduced by Yang in 2016, as a good alternative in aiding the treatment of macular hole [29]. The lens capsule is thicker and has a higher density than ILM, making it easier to place on the targeted retinal surface [29, 30].
LAMELLAR EPIRETINAL PROLIFERATION
Optical coherence tomography is also a useful diagnostic tool in diagnosing lamellar hole-associated epiretinal proliferation (LHEP). The LHEP is located between the inner border of the ERM and the retinal nerve fiber layer [31]. Intraoperatively, it is described as a thick yellow membrane surrounding the retinal hole in a patient with RP [32]. Histologic examinations show that this tissue contains macrophages, Müller cells and fibroblasts [31]. According to Parolini et al., surgical intervention for macular hole in RP with associated LHEP remains controversial because it does not improve visual function. LHEP is a risk factor for FTMH.
EPIRETINAL MEMBRANE
The incidence of ERM in RP is 1.4-51.1% [10, 33]. Vitreoretinal surgery is a treatment option for the epiretinal membrane, but the available results indicate that the decision on the procedure should be taken carefully in patients with RP due to variable postoperative outcomes [34, 35]. After the surgical treatment, a good anatomical result was generally obtained, but this did not improve final visual acuity [35]. In addition, it has been observed that even after uncomplicated ERM surgery, a patient with RP may develop severe central retinal degeneration [36].
VITREOMACULAR TRACTION SYNDROME
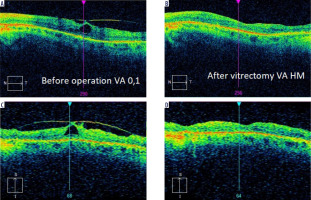
Vitreomacular traction syndrome (VMTS) in patients with RP has a prevalence of 0.6-27.3% [26]. There is evidence that patients with RP have vitreous degeneration, including vitreous gel collapse and posterior vitreous detachment, and that such lesions can cause macular complications related to vitreoretinal tractions [34]. As in cases with epiretinal membrane, the decision on surgical treatment should be taken cautiously. Figure 3 shows the case of a patient with RP treated at our Department of Ophthalmology after VMTS surgery, where visual acuity deteriorated significantly despite the morphological improvement of the central retina.
MANAGEMENT OF THE PATIENT WITH RETINITIS PIGMENTOSA
Since macular abnormalities are more common in RP compared to the general population, an OCT screening test is recommended for any patient with RP to help identify macular disease [10]. If CME is diagnosed during the tests performed, the use of CAIs should be discussed with the patient for reduction in CME and for probable maintenance of visual acuity and its slight improvement. Treatment should be closely monitored and modified by an ophthalmologist. For second-line treatment, topical steroids should be considered – either by periocular injections or as an implant [37].
The decision to surgically treat ERM and VMTS in patients with RP should be taken very carefully, informing about the possible positive as well as negative effects of the surgery. The appropriate solution in these cases seems to be to propose surgery to the patient only when the OCT image indicates significant distortion of the central retina.
In the case of FTMH, vitreoretinal surgery is the most effective treatment option, involving not only anatomical improvement of the retina after surgery, but also improved visual acuity [26].
PREVENTIVE MEASURES
From the available preventive options for patients with RP, wearing blue blocker glasses [38] and avoiding overexposure to light are recommended as this can reduce phototoxic effects on the retina. Patients and their families should be educated on and prepared for the anticipated progression of RP. Patients should be referred to local support groups and associations for the visually impaired, with the ability to select optical aids that are comfortable for them. It should be noted that due to the significant impact of the disease on the patient’s independence and well-being, their family and relatives, as well as clinicians, should be vigilant in assessing the patient’s mental status and recognizing depression, which can become a serious complication of the disease [13].
CONCLUSIONS
Macular abnormalities often occur in patients with retinitis pigmentosa. Topical carbonic anhydrase blockers and steroids can be effective in treating cystoid macular edema. Surgical treatment can improve macular morphology but improvement in visual function is limited due to long-term retinal dysfunction and progression of the underlying disease.

 POLSKI
POLSKI