Summary
Bioresorbable scaffolds (BRS) were introduced into clinical practice to overcome the limitations of metallic drug-eluting stent (DES) in terms of permanent vessel encapsulation. Initial enthusiasm for the first-generation BRS Absorb was tempered by safety concerns. In our study, we evaluated the safety and efficacy of the second-generation BRS Magmaris compared to one of the leading second-generation ultra-thin DES (Ultimaster) in the acute coronary syndrome subset at mid-term follow-up. Implantation of the Magmaris was associated with a target lesion failure rate similar to that of the novel DES, suggesting a good safety and efficacy profile for a novel device.
Introduction
Shortly after its introduction to clinical practice percutaneous coronary intervention (PCI) emerged as the gold-standard therapy for most patients with acute coronary syndromes (ACS) [1]. However, the ACS subset is a well-known risk factor for unfavorable clinical outcomes of PCI. To overcome these shortcomings we can observe continuous research efforts focused on the development of novel coronary stents. Improvements included changes in stent architecture, modification of stents’ biopolymer composition, and use of new resorbable polymers or metal alloys as part of the scaffold backbone [2].
The introduction of the first generation of drug-eluting stents (DES) into daily clinical practice marked a turning point in the field of PCI. The first generation of DES overcame the major shortcomings associated with bare-metal stent (BMS) revascularization, namely the reduction of neointimal hyperplasia, leading to a significant reduction in the need for repeat revascularization. The continued evolution of DES technology has resulted in significant reductions in strut thickness. Second-generation DES, also known as ultra-thin strut DES, have increased deliverability and significantly shortened endothelialization time, resulting in a further reduction in device-related adverse events [3]. The Ultimaster coronary stent (Terumo Corporation, Tokyo, Japan) is an ultrathin-strut, cobalt chromium, biodegradable-polymer, sirolimus-eluting stent which showed an excellent overall device safety and efficacy profile in long-term follow-up [4–6].
Plaque rupture and erosion have been shown to be the basis of the pathophysiology of the vast majority of ACS cases. Local transient exacerbation of the inflammatory process is strongly related to both ACS pathways. A novel, less invasive management of this “urgent” instability in coronary circulation has been postulated as a promising therapeutic approach. This would avoid the permanent caging of a vessel with a metallic backbone of a DES, with all the long-term consequences of this fact (transformation of the urgent local inflammatory process into a permanent inflammatory reaction to the foreign body introduced into the vessel wall). Theoretically, BRS technology enables the temporary healing of local hyperinflation associated with ACS and subsequent anatomic and functional restoration of the vessel after the resorption of the scaffold. The main idea of the BRS concept [7, 8] was to provide lumen patency in the early phase, allow vascular healing, and dissolve over time, potentially leading to a reduction of long-term device-related complications. Initial enthusiasm related to the polymer scaffold Absorb (Abbott Vascular, Santa Clara, United States) was restrained after publishing long-term outcomes [6, 9, 10].
Nevertheless, the BRS concept continues to evolve [11], and recently the magnesium bioresorbable scaffold Magmaris (Biotronik, Berlin, Germany) has been introduced to clinical practice [12]. Primary short-term data are encouraging, particularly in terms of stable CAD [13–15] and partially in the ACS subset [16–20]. Still, future evaluation of this technology is necessary to fully assess the potential gain-risk balance. In particular, clinical data beyond short-term observations (> 1 year) after Magmaris implantation in patients with ACS are still limited.
Aim
In this context, we designed this study to evaluate the long-term safety and efficiency of the Magmaris BRS in comparison to one of the leading new-generation DES, Ultimaster, in the ACS population.
Material and methods
Study design and population
A complete list of study inclusion and exclusion criteria along with a full discussion of all aspects of study design was previously published [19, 21]. Vascular calcification was assessed by angiography as mild (spots), moderate (≤ 50% of reference lesion diameter) or severe (> 50% of reference lesion diameter). Briefly, the study population consisted of patients (n = 362) with acute coronary syndrome (NSTE-ACS) who underwent PCI. Based on implanted devices patients were assigned to one of two arms (193 – Magmaris vs. 169 –Ultimaster). All PCI was performed at the Cardiology Department of the Cooper Health Center in Lubin, Poland, between October 2016 and March 2020. Additionally, all participants included in this retrospective, observational study signed written consent for PCI procedures along with later clinical evaluation. This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Medical Lower Silesian Ethics Board (number 20/07/2016, approval 12.07.2016). The study flow chart is presented in Figure 1.
PCI procedures
All PCI procedures were performed by high-volume PCI operators with extensive experience with BRS technology (performing at least 400 PCIs per year and a history of at least 50 BRS implantations before entering the study) at the Cardiology Department of the Cooper Health Center in Lubin between October 2016 and March 2020. Operators were strongly encouraged to meet the “4P” criteria during all the PCI procedures in both study arms. The clinical value of the “4P” implantation technique is well established [22, 23] and is part of contemporary practice for BRS placement [24]. The general features are based on: adequate preparation of the lesion (using a non-compliant (NC) balloon, sized with a 1 : 1 balloon to artery ratio), proper sizing, paying attention to the expansion limits, mandatory post-dilation with a non-compliant (NC) balloon with high pressure (at least 16 atm) sized 1 : 1 balloon/scaffold ratio or higher. The decision regarding support of intravascular ultrasound/optical coherence tomography (IVUS/OCT) was left to the discretion of the operators. The dual antiplatelet therapy (DAPT) in both study arms was prolonged up to 12 months after implantation in accordance with applicable guidelines ESC/ESH guidelines.
Clinical outcomes
The primary outcomes consisted of death from cardiac causes, myocardial infarction, and in-stent thrombosis. The principal secondary outcomes were device-orientated and defined as target-lesion failure (TLF), target vessel-related myocardial infarction (TV-MI), and target lesion revascularization (TLR). The TLF, TV-MI, TLR, and stent thrombosis were defined in accordance with Academic Research Consortium guidelines [25]. Diagnostic criteria of myocardial infarction met the criteria contained in the Fourth Universal Definition of Myocardial Infarction [26] Additional clinical variables were collected, including scaffold restenosis, death from any cause, cerebrovascular episodes, and need for any other revascularization procedure. All the clinical data were obtained by trained staff (physicians and/or nurses) during personal visits or telephone contact during the 1-year and 2-year follow-up periods.
Study devices
Magmaris – previously known as DREAMS 2G – is a magnesium bioresorbable metallic scaffold coated (7 μm layer) with a poly-L-lactic acid biodegradable polymer (PLLA) eluting sirolimus (1.4 μg/mm2 of the scaffold surface). The approximate time of drug release is calibrated for a 90-day, magnesium backbone resorption time, estimated for at least 1 year, while PLLA resorption lasts for 2 years. The backbone of the Magmaris device is completely radiolucent. Therefore, it has two permanent tantalum radiopaque markers mounted at the distal and proximal stent end. Magmaris is available in only 2 diameters of 3.0 and 3.5 mm and three lengths of 15, 20, and 25 mm.
The Ultimaster is a cobalt–chromium (Co-Cr) coronary stent consisting a thin strut (80 μm) coated on its abluminal side with sirolimus (3.9 μg/mm of stent length), and bioresorbable poly (D,L-lactide-co-caprolactone) copolymer (PDLLA-PCL). Both the drug and polymer are designed for a simultaneous resorption time within 3–4 months. Despite the fact that the Ultimaster DES is available in a wide range of diameter sizes (2.25–4.0 mm) and lengths (from 9 to 38 mm), for the purpose of this study in the Ultimaster cohort we used stents only with two diameters (3.0 and 3.5 mm) and lengths up to 24 mm.
Statistical analysis
Continuous variables were characterized by mean and standard deviation, while categorical variables were characterized by the frequencies. The study cohorts were compared with the nonparametric two-sample Mann-Whitney test for continuous variables and Fisher’s exact test for categorical variables. Bonferroni correction was applied to adjust for multiple comparisons. P-values ≤ 0.05 were defined as statistically significant. The log-rank test was used to compare the entire survival curve. Additionally, in terms of two major study endpoints we performed non-inferiority analysis. All statistical analyses were performed using the R language by a professional statistician familiar with medical analysis.
Results
The basic clinical characteristics of both study cohorts along with procedural features have already been already discussed and published previously. Briefly, all mentioned data are presented in Tables I and II.
Table I
Study patient characteristics
Table II
Procedural characteristics
At 1-year follow-up, no significant differences among primary and secondary endpoints between both study groups were noted. Still, in the Magmaris subpopulation in terms of the primary outcome, we observed a non-statistically significant lower rate of adverse events (1.5% vs. 5% respectively, p = 0.07). This difference was more apparent at the 2-year follow-up (5.1% vs. 11% respectively, p = 0.051) and was found to be just short of statistical significance. However, when we analyzed the second, device-oriented, composite endpoint of the study – TLF at 2-year follow-up – no significant difference was found (5.6% vs. 8% respectively, p = 0.41). In both study cohorts in terms of 2-year follow-up, no episode of stent thrombosis was observed. After 2 years of observation, we did not note any significant differences in terms of scaffold restenosis between the study cohorts (5.1% vs. 4% respectively, p = 0.81). Clinical outcomes in both study cohorts were pooled and presented in Table III.
Table III
Clinical outcomes in both study arms
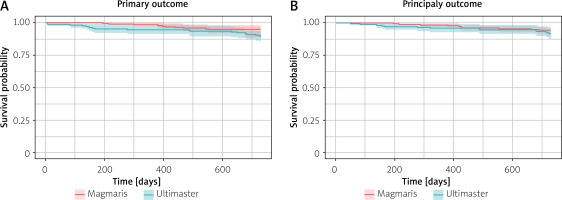
Figure 2 presents the Kaplan-Meier graph for the primary outcome and principal secondary outcome-free survival. The log-rank test was used to evaluate the primary outcome survival curve; the p-value did not reach statistical significance (p = 0.052).
Subsequently, similar analysis for the secondary endpoint was performed, and similarly the p-value did not reach statistical significance (p = 0.34), thus indicating a lack of significant difference regarding survival between Magmaris and Ultimaster.
To fully evaluate the difference between the two study cohorts, we performed a non-inferiority analysis of Magmaris stents versus Ultimaster stents in terms of primary and principally secondary endpoints. The odds ratio for the major endpoint in cases with Magmaris vs. Ultimaster was OR = 0.4593873, 95% CI (0.1836107, 1.0865151); nevertheless, the p-value for equal ratios in both groups was non-significant, which did not support rejection of the hypothesis regarding equivalence of these methods. The delta value for non-inferiority analysis was 0.1, and the analysis (using Fisher’s test) did not reach statistical significance, thus suggesting that Magmaris was non-inferior to Ultimaster.
Subsequently, the analysis for the secondary endpoint was performed. The odds ratio for Magmaris vs. Ultimaster was OR = 0.4740949, 95% CI (0.1895651, 1.1208551). However, the p-value for equal ratios in both groups was non-significant, which did not support rejection of the null hypothesis regarding equivalence of these methods. The delta value for non-inferiority analysis was 0.1, and the analysis (using Fisher’s test) did not reach statistical significance, thus suggesting that Magmaris was non-inferior to Ultimaster.
Discussion
To the best of our knowledge, this is the first study designed to evaluate the mid-term results of the novel magnesium BRS Magmaris compared to the second generation metallic DES Ultimaster in “real life” scenarios is subjects with the ACS subset. The main findings of this study are:
In our retrospective, non-randomized study cohort Magmaris showed good clinical outcomes in terms of the primary endpoint (death from cardiac causes, myocardial infarction, in-stent thrombosis) and the secondary device-orientated endpoint defined as target-lesion failure (TLF), target vessel-related myocardial infarction (TV-MI), and target lesion revascularization (TLR). Although no significant differences were observed at 2 years, the Magmaris cohort showed a borderline significant primary endpoint reduction (5.1% vs. 11% respectively, p = 0.051).
Magmaris BRS did not present any definite scaffold-related thrombosis after a 24-month observation period.
Data regarding the long-term evaluation of Magmaris BRS are rather sparse and mainly originate from retrospective observational clinical registries [20, 27–29]. Based on this single-arm evaluation despite all of its methodological shortcomings Magmaris showed favorable long-term safety and clinical performance with acceptable target lesion failure rates (5.2% up to 11%), which is comparable to our findings (5.6%) and analogous to novel DES performance [30–32]. Also in our study, we did not note significant differences between the Magmaris and Ultimaster DES in terms of TLF (5.6% vs. 8.0, p = 0.41). The lack of mid and long-term data makes the results of our study difficult to compare. However, the obtained results seem to be consistent with the findings previously reported in the short-term evaluation. Hideo-Kajita et al. [33] during a 12-month clinical follow-up noted no significant differences in TLF between Magmaris and the biodegradable polymer sirolimus-eluting stent Orsiro (6.0 vs. and 6.4%). Remarkably, the aforementioned meta-analysis suggests that Orsiro has the best 1-year performance among modern DES [30, 33–35]. Similar favorable outcomes comparing Magmaris to novel DES were also observed by Rola et al. [19, 21] and Włodarczak et al. [18]. On the other hand, Tousek et al. [36] in a small, single-center study (n = 50) evaluated in quantitative coronary angiography (QCA) and OCT performance of Magmaris compared to Xience in an ACS setting. Both QCA and OCT revealed a greater extent of late lumen loss in the Magmaris group than in the Xience group after a 12-month follow-up. These unfavorable clinical results were also observed in terms of the STEMI subpopulation. The 1-year evaluation of STEMI patients [37] treated with implantation of Magmaris or Orsiro-DES revealed a significantly higher rate of TLR in the Magmaris group (16.2% vs. 5.3%; respectively p = 0.03). Notably, implantation of the second-generation BRS (Magmaris) was associated with a significantly lower number of TLFs compared to the first-generation BRS (Absorb) (1.5% vs. 5.6%; p = 0.048) [18, 38].
The risk of stent thrombosis and repeat target lesion revascularization after PCI is higher in patients with ACS at enrollment [39, 40]. Although initial safety and efficacy trials of first-generation BRS and second-generation metallic BRS (Magmaris) focused primarily on patients with stable coronary artery disease [7, 8, 29, 33], several factors could theoretically benefit the ACS population. ACS patients are often young, with a long life expectancy and a lower incidence of previous coronary intervention with DES use, which could potentially promote vessel healing after BRS implantation. In addition, culprit unstable ACS lesions are generally composed of soft, easily expandable plaque, which appears to be conducive to the “4P” implantation strategy so critical to optimal BRS deployment [22–24]. Moreover, the higher strut thickness of the BRS compared to metallic DES may be beneficial in the process of thrombus entrapment under the struts after scaffold implantation, and it may be reflected in the reduction of the distal flow impairment [41–43]. On the other hand, the ACS subset per se increases stent thrombosis rates [44]. This, combined with the previously described trend toward higher scaffold thrombosis rates with first-generation BRS [9, 45, 46], may negate any theoretical benefit of BRS. However, this was not confirmed in a pooled meta-analysis [47]; nevertheless, the increased rate of device-related endpoints led to restrained commercial use of the first-generation BRS. On the other hand, short-term data on the use of Magmaris in the ACS subpopulation are encouraging [16–21]. However, recently published long-term data [48] suggest that the subgroup of NSTEMI patients had a significantly higher rate of TLF after implantation of the Magmaris BRS compared to non-NSTEMI patients (9.3% vs. 6.2%, p = 0.025). Yet, the results of our study did not confirm this observation, as the TLF rate of 5.6% was similar to the non-ACS cohort (6.2%) [46].
This study has several limitations. The study has a retrospective non-randomized observational character. The study population is relatively small compared to leading DES studies, but still, in terms of “real-life” BRS studies is noticeably high. Finally, despite the study population consisting of NSTE-ACS patients, we observed a relatively low number of intravascular guidance PCI procedures.
Conclusions
In our study population (NSTEMI-ACS) implantation of a second-generation BRS (Magmaris) in comparison to therapy with a novel ultrathin second-generation DES (Ultimaster) was associated with a similar rate target lesion failure in terms of 2-year follow-up. Furthermore, none of the thrombotic safety concerns occurred in either study cohort.