Introduction
Diverticulosis is an anatomical condition with an increasing incidence as the population ages. Diverticula are estimated to be the most common non-cancerous finding during screening colonoscopies, making diverticulosis a challenge in terms of both diagnosis and treatment in everyday clinical practice, as well as a significant burden on healthcare systems [1].
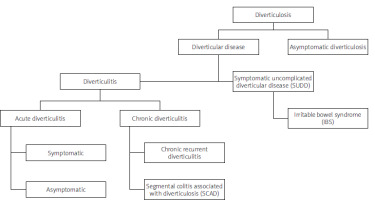
Diverticulosis is rarely diagnosed before the age of 40 years, but it is more commonly seen in about 60% of people over the age of 70. The condition may be asymptomatic and discovered incidentally during endoscopic examinations, or it may present with symptoms that lead patients to seek medical attention. These symptoms can arise from acute conditions, such as inflammation of one or more diverticula or bleeding from a vessel within a diverticulum. Additionally, diverticulosis may cause chronic gastrointestinal symptoms, such as abdominal pain, bloating, or changes in bowel habits, which are collectively known as symptomatic uncomplicated diverticular disease (SUDD). Following an episode of diverticulitis, functional disorders resembling irritable bowel syndrome may also develop. Less commonly, patients with diverticula develop segmental colitis, a condition that closely resembles inflammatory bowel disease. This condition, known in medical terminology as segmental colitis associated with diverticulosis (SCAD), can sometimes be difficult to distinguish from other forms of inflammatory bowel disease [2]. The pathogenesis of diverticular disease is multifactorial and not fully understood. Factors such as the structure of the colon wall, colonic motility, genetic predispositions, fibre intake, vitamin D levels, obesity, and physical activity are all considered to play a role in its development [2, 3].
Given the broad clinical spectrum of diverticulitis, ranging from asymptomatic diverticulosis (AD) to SUDD, uncomplicated diverticulitis, and ultimately complicated diverticulitis, clinical management and therapeutic approaches vary depending on the severity of the disease. In general, management focuses on preventing disease progression, treating active disease, and preventing relapse.
Asymptomatic diverticulosis does not require drug treatment. Instead, recommendations include adopting a healthier lifestyle, such as engaging in regular physical activity, maintaining a healthy weight, and avoiding smoking. Additionally, a diet rich in fibre can help prevent disease progression. Most people with diverticula (80–85%) do not experience symptoms, while 15–20% may report symptoms without inflammation. However, some individuals may experience complications [4–6].
Research has shown that diet plays a significant role in the development and management of diverticulosis. Consuming red meat and having a low fibre intake can increase the risk of diverticulitis. Analysis of nutritional factors as a risk factor suggests that the diet currently recommended for the prevention of cardiovascular and other chronic diseases also has a beneficial effect on the clinical course of diverticulosis. Furthermore, introducing short-term dietary interventions can reduce the risk of diverticulitis in individuals with diverticula [7–11].
Aim
The aim of this study was to assess dietary patterns among patients with diverticulosis and their influence on the symptoms presented, based on the adopted classification (Figure 1).
Material and methods
A total of 946 colonoscopies performed in the Department of Gastroenterology to diagnose reported abdominal complaints were analysed. Of these, 331 endoscopic examinations (representing 35% of the study group) resulted in a diagnosis of diverticulosis. Patients with diagnosed diverticulosis were subsequently examined using a 24-hour nutritional interview questionnaire and the FFQ-6 form to assess the frequency of consumption of specific products.
After evaluating the completed forms for quality and completeness, 100 patients (71 women and 29 men) aged between 40 and 90 years were selected for further analysis. Patients were classified according to the diverticular disease classification based on colonoscopy findings, clinical history, and Roman IV criteria (Figure 1).
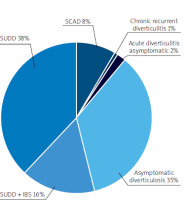
Dietary analysis was conducted for each diverticular disease group using the FFQ-6 form and the 24-hour nutritional interview. Patients with acute diverticulitis and chronic recurrent diverticulitis were excluded from the analysis due to an insufficient number of participants (Figure 2).
Statistical analysis
The assumption of normality for the compared groups was assessed using the Shapiro-Wilk test and half-normality graphs. To test the significance of differences in the average levels of unrelated variables, the parametric ANOVA test for independent samples was employed. If the hypothesis of equal variances was rejected, a Tukey RIR post-hoc test was performed to determine which specific pairs showed significant differences. Statistically significant differences are illustrated with frame charts showing means, standard errors, and 95% confidence intervals. The level of significance was set at 0.05. The calculations were conducted using the Statistica 13.0 statistical package (Dell Statistica), and the charts were prepared in Microsoft Excel 2010. The study was approved by the Independent Bioethics Commission for Research, approval number NKBBN/25/2019.
Results
In our research, we analysed 100 patients with diverticula identified during colonoscopic examinations, subjecting them to both clinical and nutritional analyses. The study group was evaluated using the instruments described in the Material and Methods section, resulting in the following outcomes (Figures 3, 4).
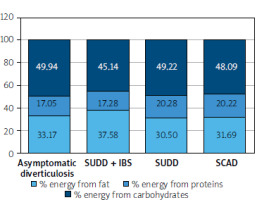
Within the study group, the distribution of disease types was as follows: the most common was SUDD at 38%, followed by AD at 35%. We analysed the energy components of the diet in the different groups with diverticular disease, as well as their consumption of micro- and macronutrients (Figure 5).
The recommended daily distribution of energy from macronutrients is as follows: protein should provide 10–20% of energy, fat should provide 20–35% of energy, and carbohydrates should provide 45–65% of energy [12]. In our study, we observed excessive energy intake from fat, particularly in the group of patients classified as SUDD. These patients also had the lowest carbohydrate intake, contributing only 45.14% of their daily energy needs. Other patients met their macronutrient requirements within the recommended standards.
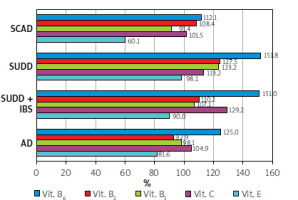
The lowest intake was observed in the SCAD group, particularly for vitamin E, which was at 60.1% of the recommended level. In contrast, the intake of B vitamins was generally above the recommended levels, ranging from 91% to 151% of the daily requirement.
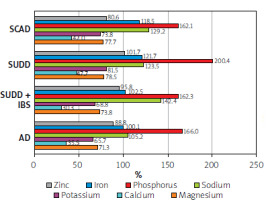
Regarding vitamins, the lowest intake of vitamin E was observed in the SCAD group. In terms of minerals, the entire study group showed insufficient calcium consumption, while the AD and SCAD groups had the lowest intake of potassium and magnesium, along with slightly lower zinc intake (Figures 6, 7).
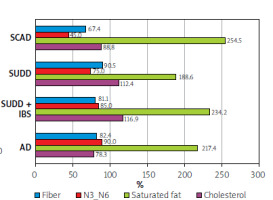
Fibre intake was approximately 30% below the daily requirement across nearly all diverticular disease groups. Additionally, there was a 2-2.5-fold higher intake of saturated fats and an imbalanced ratio of omega-3 to omega-6 fatty acids, particularly noticeable in the SUDD group (Figure 8).
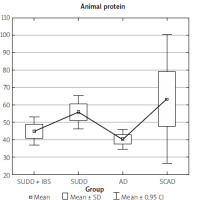
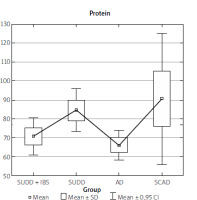
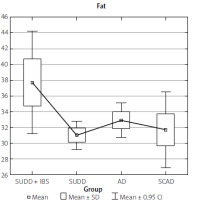
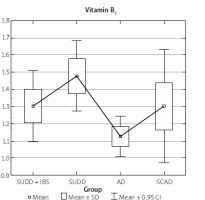
The data collected from the FFQ-6 form and the 24-hour nutritional interview were statistically analysed, yielding the results presented in Table I. The analysis results indicate that the SUDD, SCAD, and AD groups differed significantly in terms of fat, cholesterol, total protein, animal protein, and vitamin B2 intake (Table I).
Table I
Statistical analysis of diet ingredients
Upon examining the individual statistical relationships, we find that the differences in animal protein intake and total protein intake (regardless of source) were significant only between the SUDD and AD groups (Figures 9, 10). Similarly, differences in cholesterol intake were observed only between the SUDD and AD groups.
The SUDD group showed significantly higher consumption of both fat and vitamin B2 (Figures 11, 12).
A theoretical discriminatory model based on a group of 9 variables has been developed, as shown in Table II.
Table II
The discriminatory model based on a group of 9 variables (n = 98)
A nutritional model that could affect the development of symptomatic diverticular disease was created based on data from interviews and questionnaires. The following dietary components were identified as determinants: 1) animal protein, 2) fats, 3) cholesterol, 4) zinc, 5) sodium, 6) vitamin B6.
Discussion
Diverticulosis is a common anatomical condition that increases in incidence with age. It is the most frequent non-cancerous finding during screening colonoscopies. While diverticulosis can be asymptomatic, it may also cause symptoms such as SCAD or SUDD, or lead to complications like diverticulitis with abscess formation, perforation, or bleeding from the lower gastrointestinal tract. In our study, we found that among those who underwent colonoscopy due to abdominal discomfort and were diagnosed with diverticulosis, the majority (38%) suffered from SUDD.
The aetiopathogenesis of diverticulosis remains a topic of debate, with many factors, including diet, being considered as potential determinants. A proper diet to meet daily energy needs should consist of 10–20% protein, 20–35% fat, and 45–65% carbohydrates. It is also recommended that saturated fats constitute less than 10% of total fat intake [12].
The main sources of saturated fatty acids in the diet include red meat, animal fats, offal, yellow cheese, processed cheese, cream, commercially prepared confectionery products, and foods containing palm oil.
Our study revealed that the SUDD group had an excessive energy intake from fat (37.58%). These patients also consumed the least amount of carbohydrates, covering only 45.14% of their daily energy requirements. Additionally, cholesterol consumption in this group was above the recommended levels; by comparison, the cholesterol intake in the AD group was 78.3% of the recommended norm.
The recommended daily cholesterol intake is up to 300 mg, with animal products being the primary sources. Cholesterol plays a crucial role in constructing cell membranes and myelin sheaths. However, excessive consumption can lead to elevated blood cholesterol levels [12].
For many years, it has been understood that blood cholesterol levels are influenced more by the consumption of saturated fatty acids than by cholesterol-rich foods [13]. Research has shown that elevated blood cholesterol levels can precede the proliferation of intestinal cells, increasing the risk of colorectal cancer [14]. Our study found that patients with diverticulosis consumed more than twice the recommended amount of saturated fats.
On the other hand, polyunsaturated fatty acids, particularly those from the omega-3 group, have demonstrated anti-inflammatory properties that can suppress excessive immune responses and reduce cellular damage. This is evident in the reduction of pro-inflammatory markers such as C-reactive protein (CRP), interleukin-6 (IL-6), and tumour necrosis factor-α (TNF-α) [15]. Increased intake of omega-3 fatty acids relative to omega-6 in the diet may lower the incidence of chronic inflammatory diseases, including rheumatoid arthritis, ulcerative colitis, Crohn’s disease, systemic lupus erythematosus, and psoriasis. The recommended omega-3 to omega-6 ratio is 1 : 5 (0.2), which should be achieved through a variety of dietary sources [16].
In our study, the most favourable omega-3 to omega-6 ratio was observed in the AD group, with a ratio of 0.18, while the SCAD group exhibited a pro-inflammatory ratio of 0.09. This suggests that an imbalance in these fatty acids may contribute to the development of intestinal diseases.
The role of dietary fibre in intestinal diseases such as diverticular disease has been widely studied, but the results are inconclusive. Some studies suggest that increased fibre intake can reduce pain, while others show no effect. Nevertheless, a high-fibre diet remains the primary nutritional recommendation for patients with diverticular disease without inflammation.
Dietary fibre refers to the indigestible components of plant cells that resist digestion by human enzymes. Fibre is categorised into 2 types based on water solubility: soluble and insoluble. The insoluble fraction includes cellulose, lignins, and most hemicelluloses, while the soluble fraction comprises β-glucans, resistant starch, pectins, fructo-oligosaccharides, and plant gums [12]. For individuals with AD, a diet rich in plant fibres, particularly insoluble fibre, is recommended. An optimal high-fibre diet consists of approximately 40–60 g of fibre daily.
Fibre increases faecal bulk, promotes bowel movement through mechanical stimulation, and aids in water absorption, thereby improving stool consistency. By optimising intestinal function, it increases bowel movement frequency and helps prevent constipation [11, 12]. Foods rich in insoluble fibre include whole-grain cereal products, bran, whole-grain flour, whole-grain bread, coarse grain groats, brown rice, and certain fruits and vegetables such as green peas and black currants. Maintaining a diverse diet with significant fibre content from various sources – including vegetables, fruits, and cereal products – helps reduce the risk of diverticulitis. It is not advisable to consistently eliminate nuts, seeds, and fruits with pits from the diet as a strategy to lower the risk of developing diverticulitis [17].
In our study, none of the groups met their daily fibre requirements, with the lowest levels of dietary fibre observed in the SUDD and SCAD groups.
Regarding dietary micronutrients, several that influence intestinal motor activity and water-electrolyte balance were considered. In particular, we assessed the daily consumption of magnesium. The primary sources of magnesium include cereal products, legumes (peas, beans, soybeans, chickpeas, lentils), nuts (walnuts, hazelnuts, almonds, pumpkin seeds), cocoa, dark chocolate, rennet cheese, fish, potatoes, bananas, and green vegetables (spinach, broccoli, parsley, green beans). The recommended daily intake of magnesium is 320 mg for women and 420 mg for men [12]. Studies indicate that consuming 40–80 mmol of magnesium can increase intestinal motility within 6 h, making it one of the recommended treatments for constipation [18–21]. Considering that constipation is a risk factor for diverticulosis, adequate magnesium intake can have a significant impact. However, none of the groups in our study met the daily required intake for magnesium, with their intake covering only about 80% of the daily requirement.
Similarly, the daily calcium intake was considerably lower than recommended, with most groups falling below 50% of the recommended intake. This includes the SUDD group, which covered only 31.6% of the recommended intake. The main sources of calcium include vegetable oils such as sunflower oil and safflower oil, as well as cereal products, dairy products, avocados, nuts, seeds (pumpkin seeds, sunflower seeds, sesame seeds, almonds), and vegetables (parsley, lettuce, spinach, chives, kale, broccoli) [12].
Similarly, there was a reduced intake of potassium, contrasting with a significantly exceeded intake of sodium. The recommended daily potassium intake is 3500 mg. Potassium plays a crucial role in maintaining proper water-electrolyte balance and regulating cellular osmotic pressure. Its primary functions include activating numerous body enzymes and participating in the metabolism of nutrients such as carbohydrates and proteins. The main dietary sources of potassium include dried fruits, nuts and seeds, cocoa, legumes (beans, soybeans, chickpeas, lentils, peas), vegetables (potatoes, broccoli, carrots, beetroots, spinach), fruits (bananas, peaches, apricots, cherries), and cereal products (buckwheat, barley, millet, brown rice, oatmeal) [12].
The recommended daily sodium intake ranges from 1200 to 1500 mg, depending on age. Sodium deficiencies can lead to weakness, nausea, vomiting, and headaches. On the other hand, excessive sodium intake is associated with hypertension, cardiovascular disease, and obesity. The primary sources of sodium in the diet are bread, meat products, dairy products, and vegetables.
In our analysis of consumed micronutrients, it is noteworthy that phosphate intake was almost doubled (1.5–1.8 times the recommended amount). The recommended daily intake of phosphorus is 700 mg. This element is present in many food products, so deficiencies are rarely observed. The main sources of phosphorus include dairy products, cereal products, and phosphates added to meat preparations, sweets, and carbonated drinks [12]. Such high consumption in the study group suggests a correlation with improper dietary habits.
No iron deficiency was observed in the study group’s diet. However, we did observe a reduced zinc intake (80% of the daily recommended amount) specifically in the SCAD group. Iron’s primary role is in erythropoiesis, with key dietary sources including animal products, legumes, leafy vegetables, sesame seeds, and almonds. Zinc plays a crucial role in maintaining membrane stability, perception of taste and smell, alcohol metabolism, and immune defence. It may also indirectly influence synaptic plasticity, memory, learning processes, energy production, and signal conduction. Zinc’s main dietary sources include meat, offal, rennet cheese, and whole-grain cereals that are directly milled.
Our study found that, aside from a reduced intake of vitamin E (down to 60.1%) in patients with SUDD, no significant vitamin deficiencies were observed. However, an excessive intake of B vitamins was noted. Vitamin E is a fat-soluble antioxidant that helps protect against cancer, coronary heart disease, and atherosclerosis. It is essential for maintaining the proper function of reproductive organs in both men and women and prevents the oxidation of polyunsaturated fatty acids. The recommended daily intake of vitamin E is 10 mg for men and 8 mg for women, which can be sourced from butter and dairy products.
The recommended daily intake of vitamin B1 is 1.1 mg for women and 1.3 mg for men. This vitamin plays a key role in numerous enzymatic reactions, facilitates nerve signal conduction, and activates chloride channels. Its main sources include meat, meat preparations, legumes, and whole grain cereal products. Vitamin B2 is involved in oxidation and reduction reactions, with the best sources being dairy products, eggs, and offal. Vitamin B6, along with its active forms – pyridoxal and pyridoxamine – acts as a cofactor for over 100 enzymes, mainly involved in amino acid metabolism, glycogenolysis, gluconeogenesis, heme biosynthesis, and neurotransmitter biosynthesis (including γ-aminobutyric acid, dopamine, and noradrenaline). Vitamin B6 is essential for homocysteine transformation, and its main sources include fish, meat, legumes, nuts, and whole-grain cereal products.
Our study identified statistical differences in the amount of protein consumed between the SUDD and AD groups, with higher intake in the symptomatic diverticular disease group. Similarly, differences were observed in the intake of vitamin B2, fat, and cholesterol.
This study has several limitations that should be acknowledged. Firstly, the sample size was limited to 100 patients, which may constrain the generalisability of the findings. A larger and more diverse sample could provide more statistically significant results and reduce potential biases. Secondly, the selection of participants from a specific hospital department may introduce selection bias, limiting the external validity of the study. Additionally, the reliance on self-reported dietary data through the Form FFQ-6 and the 24-hour nutritional interview presents another limitation, as these methods are susceptible to recall bias, potentially affecting the accuracy of dietary assessments. The cross-sectional design of the study further limits the ability to establish causality between dietary patterns and diverticular disease. A longitudinal approach would be more suitable to determine the directionality of these relationships. Moreover, the study primarily focused on dietary factors, with limited consideration of other potential confounders such as physical activity, genetic predispositions, and comorbidities, which could also influence the development and progression of diverticular disease. The geographical and cultural context of the study may also limit the applicability of the findings to other populations with different dietary habits. The study identified insufficient intake of certain nutrients like calcium, potassium, magnesium, and zinc, which could be due to either limitations in the dietary assessment tools used or the actual dietary patterns of the participants. This limitation may affect the accuracy of conclusions regarding the role of these nutrients in diverticular disease. Lastly, the absence of long-term follow-up in the study restricts the ability to assess the impact of dietary modifications on the progression or resolution of diverticular disease over time. These limitations highlight the need for further research with a more robust design, larger sample size, and consideration of additional factors to confirm these findings and enhance the understanding of the relationship between diet and diverticular disease.
Conclusions
The most common group among the classified diverticulosis cases was the SUDD group, which exhibited the highest energy intake from fat and the lowest carbohydrate consumption. All groups reported a reduction in dietary fibre intake by at least 30%, while saturated fat consumption was 2 to 2.5 times higher than recommended. In the SCAD group, an unfavourable N3 ratio was observed, which may promote intestinal inflammation.
Excessive consumption of B vitamins was noted across the groups. Specifically, the SCAD group showed a lower intake of vitamin E, with consumption at about one-third of the recommended level. Additionally, there was insufficient intake of calcium (Ca), potassium (K), magnesium (Mg), and zinc (Zn), while sodium (Na) and phosphorus (P) intake exceeded recommended levels. This imbalance could affect the development of diverticulosis due to its effect on intestinal motility.
Analysis of the nutritional data from the questionnaires suggests that the key factors contributing to the development of diverticular disease may include high intake of animal protein, fat, and cholesterol, a deficiency of micronutrients such as zinc, excess sodium, and excessive consumption of B vitamins, particularly vitamin B6.
Recommendations
Increase vegetable consumption.
Decrease consumption of animal fats.
Maintain a proper N3 fatty acid ratio.
Increase intake of calcium, potassium, magnesium, and zinc while reducing sodium and phosphorus consumption (consider the impact of preservatives).
Increase vitamin E intake and reduce B vitamin intake, particularly vitamin B2.
Based on the findings of this study, several avenues for future research are proposed to enhance the understanding and management of diverticular disease. Future studies should focus among others on the long-term impacts of specific dietary modifications on the progression of diverticulosis into more severe forms, such as SUDD and SCAD. Longitudinal studies are necessary to assess the efficacy of these interventions in preventing disease progression and reducing complications. The imbalance in the omega-3 to omega-6 fatty acid ratio observed in patients, particularly in the SCAD group, suggests a potential role in promoting intestinal inflammation. Future research should investigate the impact of adjusting this ratio on inflammatory markers and disease outcomes in diverticulosis. Comparative studies should explore the effects of different protein sources, particularly animal versus plant-based proteins, on the incidence and progression of diverticular disease. This could lead to more refined dietary guidelines for at-risk populations. To better understand the influence of genetic, environmental, and lifestyle factors on the relationship between diet and diverticulosis, cross-population studies should be conducted. These studies would help identify population-specific risk factors and inform tailored dietary and therapeutic interventions. These research directions aim to refine dietary recommendations and improve the management of diverticular disease through personalised nutrition and targeted interventions.