Introduction
Carotenoids are pigments naturally found in subcellular structures called chloroplasts of photosynthetic organisms, as well as certain bacteria and fungi. Several carotenoids have been characterized to date (Rebelo et al., 2020). These compounds are considered major pigment categories and are produced as secondary metabolites in various fruits, vegetables, and a few microbes (Bera, 2019). Carotenes are a class of hydrocarbons that are not combined with other elements, constituting less than 10% of the total carotenoid species. On the other hand, xanthophylls are characterized by the presence of oxygen atoms, and their side-chain derivatives may include hydroxy-, keto-, methoxy-, epoxy-, or carboxyl groups (Mordi et al., 2020). Canthaxanthin, a prominent keto-carotenoid, is found in various plants and is responsible for the coloring of fruits, vegetables, and flowers. It is also present in green algae, bacteria, crustaceans, fish, and vertebrates (Bera, 2020). Canthaxanthin exhibits potent oxygen-quenching and free radical deactivating properties (Dewanjee et al., 2021). The biological synthesis of canthaxanthin occurs through the activity of β-C-4 oxygenase (β-carotene ketolase), an enzyme involved in catalyzing the conversion of β-carotene into canthaxanthin (Rebelo et al., 2020). Canthaxanthin has the molecular formula C40H52O2, a molar mass of 564.84 g/mol, and the CAS number 514-78-3. This symmetrical ketocarotenoid contains two conjugated carbonyl groups, and its spectrum exhibits a broad symmetrical peak with a maximum at 466 nm in petroleum ether (%III/II = 0) (Rodriguez-Amaya, 2001). While canthaxanthin can be synthesized artificially, there is a growing demand for naturally derived canthaxanthin and other commercially valuable carotenoids obtained from plants and various other microbes. The global market for carotenoid pigments has been steadily increasing due to consumer preference for naturally sourced active components over synthetic products (Torregrosa-Crespo et al., 2018).
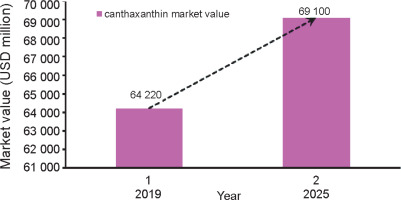
In 2018, the global carotenoid industry was valued at approximately USD 1.40 billion. It is projected to reach USD 1.85 billion by the end of 2026, with a compound yearly growth rate of 3.57%. Between 2007 and 2017, there was a significant 1500-fold increase in the utilization of carotenoids worldwide. In the commercial food market, carotenoids accounted for 1609.8 metric tons, while nonfood-based products accounted for 63.0 metric tons (Yaqoob et al., 2021). A recent study suggests that there will be a notable surge in global canthaxanthin trade, with an expected growth from USD 64220 million in 2019 to USD 69100 million by 2025, representing a compound annual growth rate of 1.85% (Fig. 1) (https://www.wboc.com).
Fig. 1
Prospective growth of the global canthaxanthin market between the years 2019 and 2025 (https://www.wboc.com)
The canthaxanthin market is experiencing continuous growth primarily driven by the increasing demand for a rational and regular source of coloring components.
The market expansion is attributed to factors such as the widespread availability of canthaxanthin, its various medical benefits, and its established role as a standard coloring agent.
Canthaxanthin holds a significant share of the carotenoid market, thanks to its extensive use as a coloring agent in feed supplements, food additives, personal care products, and pharmaceutical applications (https://www.globenewswire.com/news). Currently, there are several commercially available canthaxanthin products on the market (Table 2).
Table 1
Various significant aspects of canthaxanthin.
https://www.verifiedmarketresearch.com/product/canthaxanthin-market https://www.emedicinehealth.com/
Table 2
Companies associated with the commercial production of canthaxanthin
| Company | Commercialized product | Country of origin | URL |
|---|---|---|---|
| BASF | Lucantin® red | Germany | https://nutrition.basf.com |
| Allied Biotech | Canthatene® Canthaxanthin | Taiwan | https://www.altratene.com |
| Dynadis SARL | MIXDYN® | France | https://www.dynadis.com |
| Ardap Care GmbH | Quiko Intensive Red | Germany | https://ardapcare.com |
| Ardap Care GmbH | Quikocarophyll | Germany | https://ardapcare.com |
| The Nekton GmbH | Nekton-R | Germany | https://allbirdproducts.com |
| Taiyo Group | Petslife Intensive Red | India | https://www.taiyogroup.in |
| DSM | Carophyll® Red 10% | USA | https://www.dsm.com |
| ZMC | Canthaxanthin Beadlet 10% | USA | https://www.ulprospector.com |
| Novepha | Sell Canthaxanthin | China | https://detail.en.china.cn |
| Parchem | Canthaxanthin | USA | https://www.parchem.com |
This article provides an extensive review of recently discovered sources of canthaxanthin and their vital applications in pharmacology and as feed additives. Special attention is given to the utilization of canthaxanthin and other carotenoids as feed ingredients for fish and poultry, considering their growing market importance. The article offers a comprehensive explanation of the past, present, and future perspectives of canthaxanthin.
Structure and isomerism of canthaxanthin
Carotenoids, from a structural perspective, exhibit a geometric pattern where two residual parts are connected by a double bond, either in the same direction (forming the E form) or in the opposite direction (forming the Z form) with respect to the plane (Moldenhauer et al., 2023). Isomers, aside from having differences in melting points, stability, and solubility, also display significant differences in absorption affinity, color, and color intensity (Venugopal, 2008).
A canthaxanthin molecule consists of a polyene chain comprising 40 carbon atoms, with double bonds and two unreacted hydroxyls (OH) groups located at each terminal ring. Notably, there is an extended double-bond system centrally positioned in the molecule. The electron density is higher towards the terminal end of the chain, while β-electrons are delocalized throughout the entire polyene chain (Bart and MacGillavry, 1968). By modifying the polyene skeleton of canthaxanthin through processes such as cyclization, atom rearrangement, chain shortening, hydrogenation, oxygen incorporation, or dehydrogenation, a wide range of structures can be generated. The β-rings of canthaxanthin form dihedral angles of 43º at their ends. The two keto groups in a canthaxanthin molecule are approximately 2.7 nm apart (Bart and MacGillavry, 1968).
Canthaxanthin is readily soluble in small volumes of water, lipids, or a mixture of organic solvent and water, and it can form molecular aggregates. In nature, all-trans-canthaxanthin, along with 9-cis- and 13-cis-canthaxanthin, has been reported to occur. Typically, HPLC is used for the separation and quantification of canthaxanthin isomers, while the technique of High-performance liquid chromatography-Atmospheric pressure chemical ionization-Mass spectrometry (HPLC–APCI–MS) is a powerful tool for confirming the presence of canthaxanthin (Schlatterer and Breithaupt, 2006). The isomerization process of canthaxanthin has gained increasing importance, and various factors such as temperature, acid, and ions have been found to influence it (Qiu et al., 2014). High temperature and light are known to influence the isomerization of carotenoids, transforming them into cis forms, which can also alter their biological functions (Bera and Dutta, 2017). Successful separation of 13 canthaxanthin isomers has been achieved using a column consisting of calcium hydroxide, and the identification of seven isomers has been accomplished using the proton nuclear magnetic resonance (1H-NMR) technique (Hashimoto et al., 1988). Among the isomers, 9-cis-canthaxanthin exhibits higher proapoptotic activity compared to all-trans-canthaxanthin (Venugopalan et al., 2013). Modern methods for the separation of canthaxanthin isomers or impurities involve the use of C30 and Si60-HPLC–APCI–MS combined with a diode array detector (DAD), which yields satisfactory results (Qiu et al., 2014). Thus far, some of the identified isomer compositions using these approaches include 9,13'-di-cis-, 13,15-di-cis-, 9,15-di-cis-, 15-cis-, 13-cis-, 9,13-di-cis-, all-trans-, 11-cis-, 7-cis-, and 9-cis-canthaxanthin (Qiu et al., 2014).
Synthesis of canthaxanthin
Chemical synthesis
Chemical synthesis of carotenoids, including canthaxanthin, involves several important factors such as procedure complexity, availability of new chemical routes, potential immune-boosting effects, and undesired side effects upon consumption (Ausich, 1997). A commonly used petrochemical-based material called “ketoisophorone” serves as a precursor for synthesizing xanthophylls. Among natural carotenoids, canthaxanthin and eight other carotenoids are utilized for large-scale industrial production (Ernst, 2002).
Canthaxanthin was historically produced commercially from β-carotene, following the method initially conceived by Karrer. The process involves allylic bromination of β-carotene, followed by solvolysis to form 4,4!-bis acetate. Subsequent hydrolysis and oxidation reactions lead to the formation of canthaxanthin (Rosenberg et al., 1979). In the late 1980s and early 1990s, Wittig olefination became an important technique for synthesizing polyenes, including carotenoids, despite the formation of unwanted by-products. Though carbon–carbon double bond formation remains a harsh but probing challenge in carotenoid synthesis, new methods were established like the aldehyde–sulfone route, to meet market demand as well as to avoid complexity (Bernhard and Mayer, 1991).
A novel method for canthaxanthin synthesis was described, involving the oxidation of 4,4′-dihydroxyl-β-carotene, which was synthesized from the condensation of C15-phosphonate 2 with C10-trienedial 3. The critical intermediate, C15-phosphonate 2, was synthesized in four steps using α-ionone as the starting compound (Pi et al., 2020). In another study, canthaxanthin was synthesized from β-carotene, and its properties were evaluated by synthesizing three different diodes using various combinations of carotene, canthaxanthin, silicon, and aluminum (Uzun et al., 2022).
Biological synthesis
In order to address growing environmental concerns and increase consumer awareness, the process of chemical synthesis, which often involves creating new isomers, is being scrutinized (Scaife et al., 2012). The health concerns associated with synthetic carotenoids have led to rising demand for naturally produced alternatives (Saini et al., 2019). One significant alternative method for canthaxanthin production is the utilization of various microbial species, offering a more sustainable approach compared to chemical synthesis (Chen et al., 2021). Exploring the secondary metabolic pathways of microorganisms can unveil new structural metabolites that can be produced at a minimal cost. Chlorella zofingiensis, a microalga, is widely used for canthaxanthin production. Additionally, the bacterium Gordonia jacobea is employed for industrial-scale canthaxanthin production (https://www.bccresearch.com/title). Moreover, noncarotenogenic microbes such as Blakeslea trispora, Saccharomyces cerevisiae, and Escherichia coli are being genetically engineered for the commercial production of carotenoids (Saini et al., 2019).
Canthaxanthin, an isoprenoid compound, is synthesized through the conversion of two basic isoprene compounds, namely isopentenyl diphosphate and dimethylallyl diphosphate. These compounds undergo condensation with geranyl diphosphate to form a C10 compound. Subsequently, this C10 compound reacts to produce geranylgeranyl pyrophosphate, a type of pyrophosphate (Wang et al., 2007). Cyclic carotenoids such as zeta-carotene, neurosporene, and lycopene are formed through the action of various desaturase enzymes. Enzymes located in the cellular membrane, such as phytoene desaturase and lycopene β-cyclase, catalyze successive steps in the synthesis of colored carotenoids, leading to the abundant formation of β-carotene (Tian and Hua, 2010).
The biosynthetic pathway for canthaxanthin production involves the enzymatic conversions of precursor molecules. The enzymes β-carotene ketolase (BKT) and β-carotene hydroxylase play crucial roles in these conversions.
In the case of canthaxanthin, β-carotene is converted to canthaxanthin through a series of steps. Initially, β-carotene is converted to echinenone by the action of β-carotene ketolase. β-Carotene ketolase introduces keto groups at C4 with or without hydroxylation at the C3 position. Echinenone is then further modified to form canthaxanthin through additional oxidations.
Another pathway involving β-carotene hydroxylase leads to the production of zeaxanthin. β-Carotene is converted to zeaxanthin via an intermediate called β-cryptoxanthin through the action of β-carotene hydroxylase.
High-yielding microalgae such as Haematococcus pluvialis (for astaxanthin) and Chlorella zofingiensis (for canthaxanthin) have been extensively studied for their commercial production of carotenoids. These microalgae possess well-characterized biosynthetic pathways that allow for the efficient production of specific carotenoids (Saini et al., 2019). The biosynthetic pathway for canthaxanthin production is depicted in Figure 2.
Sources of canthaxanthin
Microbial sources
Isolation of canthaxanthin was first reported in the mid-19th century from the edible mushroom Cantharellus cinnabarinus (Haxo, 1950). Studies have shown that canthaxanthin is produced as a secondary carotenoid in several green algae, blue-green algae, and bacteria. It is synthesized either at the end of the growth phase instead of or in addition to primary carotenoids (Hertzberg et al., 1966; Saperstein et al., 1954).
In recent years, researchers have been increasingly interested in microbial sources of canthaxanthin due to their advantages over other natural sources. Microbial fermentation processes can be easily controlled to achieve higher growth rates and greater cell density, offering the potential for efficient canthaxanthin production (Das et al., 2007; Krupa et al., 2010). Moreover, microbial production of canthaxanthin does not pose significant limitations in terms of space and time, making it a promising approach for commercial production (Bhosale and Bernstein, 2005).
While there is limited information available regarding the commercial-scale production of canthaxanthin by various microbes, there is a wide range of microbial sources that have been identified as potential producers of canthaxanthin are listed in Table 3.
Table 3
Various microbial sources of canthaxanthin
| Organism name | Type | Reference | |
|---|---|---|---|
| Bacteria | Dietzia maris | bacteria | (Venil et al., 2021) |
| Bradyrhizobium sp. Strain ORS278 | bacteria | (George et al., 2020) | |
| Bradyrhizobium sp. | bacteria | (Agarwal et al., 2023) | |
| Escherichia coli | bacteria | (Madhavan et al., 2022) | |
| Gordonia jacobaea | bacteria | (de Miguel et al., 2001) | |
| Dietzia natronolimnaea HS1 | bacteria | (Nasri et al., 2010a) | |
| Micrococcus roseus | bacteria | (Karbalaei-Heidari et al., 2020) | |
| Brevibacterium sp. | bacteria | (Mitra et al., 2021) | |
| Rhodococcusmaris | bacteria | (Nelis et al., 1991) | |
| Corynebacterium michiganense (mutant) | bacteria | (Watcharawipas et al., 2022) | |
| Dietzia sp. K44 | bacteria | (Venugopalan et al., 2013) | |
| Algae | Chlorella emersonii | algae | (Cezare-Gomes et al., 2019) |
| Chlorella zofingiensis | algae | (Pelah et al., 2004) | |
| Dictyococcus cinnabarinus | algae | (Rather et al., 2022) | |
| Coelastrella striolata var. multistriata | microalga | (Abe et al., 2007) | |
| Haematococcus lacustris | algae | (Chattopadhyay et al., 2008) | |
| Scenedesmus sp. | microalgae | (Rajput et al., 2022) | |
| Nostoc punctiforme PCC 73102 | algae | (Llewellyn et al., 2020) | |
| Others | Cantharellus cinnabarinus | fungus | (Martinez-Camara et al., 2021) |
| Haloferax alexandrines Strain-TM T | archaeon | (Asker et al., 2002) | |
| Mucor circinelloides (genetically modified) | fungi | (Naz et al., 2021) | |
| Monascus roseus | yeast | (Dufosse et al., 2009) |
Higher animal sources
Canthaxanthin is naturally produced in many mushrooms, and it can also be found in the eggs of fishes and crustaceans (https://www.cfs.gov.hk). The presence of canthaxanthin has been established in various fish species, including carp Cyprinus carpio [family: cyprinidae] (Katayama et al., 1973), golden mullet (Mugil auratus) [family: mugilidae], Diplodus annularis [family: sparidae], and Crenila brustinca [family: labridae] (Czeczuga, 1973). However, it is worth noting that canthaxanthin is not located in wild Atlantic salmon, although a minor portion of carotenoid was found in wild Pacific salmon (Caballo et al., 2012). Canthaxanthin has also been found in the wild trout Salmo trutta (Briers et al., 2013) and in the bird golden-crowned kinglet (Chui et al., 2011).
Genetically modified organisms as canthaxanthin sources
Importance of canthaxanthin
Canthaxanthin as a natural antioxidant
Canthaxanthin has been found to possess antiaging and antioxidant capabilities, making it effective in scavenging free radicals, combating oxidative stress, and enhancing endogenous antioxidant defenses (Mathimaran et al., 2021). In vitro studies have shown that keto-carotenoids like canthaxanthin exhibit higher antioxidant and free radical scavenging capacities compared to carotene carotenoids like lycopene or β-carotene. This is attributed to the stability provided by the conjugation of the keto group with the polyene backbone, which enhances the ability to stabilize carbon-centered radicals (Esatbeyoglu et al., 2017).
Canthaxanthin acts as a strong antioxidant [reduction potential of dietary canthaxanthin in triton X micelles is 1041 {E0 (CAR•+/CAR)/mV}] (Bohm et al., 2012) owing to the location of keto groups at the 4 and 4′ positions in the β-ionone ring. The rate constant for singlet oxygen quenching for canthaxanthin is near 1.45 × 1010 mol/s (Choe et al., 2009). Canthaxanthin acts as a strong antioxidant by reacting with phenoxyl radicals generated by the one-electron metabolism of phenolic compounds, the reaction being catalyzed by the peroxidase enzyme (Tyurin et al., 1997). The antioxidant potential of canthaxanthin against photo-oxidation by energy dissipation is well known (Albrecht et al., 2001).
Comparative studies have shown that lycopene exhibits the highest radical scavenging ability among carotenoids is lycopene > β-cryptoxanthin ≈ β-carotene > lutein ≈ zeaxanthin > α-carotene > echinenone > canthaxanthin = astaxanthin (Muller et al., 2011). Similarly, another experiment ranked carotenoids in terms of stability, with α-tocopherol > lycopene ~ β-tocopherol ~ γ-tocopherol > β-carotene > zeaxanthin ~ 8-tocopherol > lutein > echinenone » canthaxanthin ~ P-apo-8′-carotenal > astaxanthin (Mortensen et al., 1997).
Storage time is critical for canthaxanthin pellets, as approximately 20% of the content can be lost over 2 months under ambient temperature. To mitigate this, natural antioxidants can be used during storage in combination with other pigments (Choubert et al., 1991). Canthaxanthin supplementation has been shown to alter α and γ tocopherol concentrations in murine tissues in a dose-dependent and time-dependent manner, with gender and tissue specificity (Nys et al., 2000). These findings indicate the involvement of canthaxanthin in cellular oxidative stress and its potential for in vivo protection against chronic diseases (Palozza et al., 1998).
Studies have indicated that the 9-cis isomers of canthaxanthin exhibit higher antioxidant activity compared to the all-trans form (Venugopalan et al., 2013). Additionally, microbial canthaxanthin produced through continuous culture systems has been found to possess greater in vitro antioxidant activity compared to canthaxanthin obtained through batch and fed-batch fermentation systems using Dietzia natronolimnaea (Gharibzahedi et al., 2013a). Supplementation of the diet with carotenoids (xanthophyll/canthaxanthin) in Chinese soft-shelled turtles has been shown to enhance antioxidant ability, reduce lipid peroxidation in serum and liver, and upregulate the expression of enzymes β-carotene oxygenase 2 (BCO2), catalase (CAT), and the superoxide dismutase 2 (SOD2) involved in antioxidant defense (Wang et al., 2021).
Canthaxanthin and lipid metabolism
Carotenoid pigments, including canthaxanthin, can influence the structural and dynamic properties of lipid membranes. Differential scanning calorimetry studies have shown that canthaxanthin can alter the thermotropic phase transition of lipid bilayers, particularly in the gel phase, but has minimal effects on phosphatidylethanolamines (Rengel et al., 2000). Canthaxanthin can restrict molecular motion in both the head groups and hydrophobic core of lipid molecules within the membrane bilayer, leading to the elongation of alkyl lipid chains. It also modifies the surface of lipid membranes, particularly in the gel state, and promotes the assembly of lipid vesicles (Sujak et al., 2005, 2009, 2012).
In terms of its impact on atherosclerosis, an independent study found that incubation of monocyte-macrophages with canthaxanthin-incorporated low-density lipoprotein (LDL) demonstrated more effective prevention of atherosclerosis compared to β-carotene and zeaxanthin (Carpenter et al., 1997). Dietary carotenoids, including canthaxanthin, have been shown to reduce the progression of atherosclerosis (Carpenter et al., 1997).
The absorption efficiency of canthaxanthin is lower in liposomes, liver microsomes, and retinal epithelial cells, although its efficacy remains unaltered (Shafaa et al., 2007). A study by Shih et al. in 2008 suggested that canthaxanthin modulates the balance between pro-oxidation and antioxidation, suppresses cholesterol-induced oxidative stress, and affects the antioxidant system and cholesterol metabolism. Canthaxanthin significantly reduces LDL-cholesterol levels in the blood by increasing the concentration of glutathione transferase and elevating the concentration of catalase in the serum and liver (Kumar et al., 2011). Canthaxanthin has also been shown to significantly increase high-density lipoprotein (HDL) levels in rats (Balasubramaniam et al., 2012).
Excessive doses of canthaxanthin in the feed of monkeys have been associated with the accumulation of canthaxanthin–lipoprotein complexes, leading to retinopathy-related diseases (Sujak, 2009). However, controlled and consistent feeding of canthaxanthin over a prolonged period can result in its accumulation in the liver, fat, lungs, and small intestine without exhibiting any toxicity (Tang et al., 1995). Excessive and prolonged consumption of canthaxanthin as a superficial skin shading agent and antitanning agent has led to the accumulation of canthaxanthin crystals inside the eye. However, these crystals were reabsorbed after discontinuing canthaxanthin consumption without causing any clinical manifestations (Mussagy et al., 2019).
Canthaxanthin and cancer prevention
Canthaxanthin exhibits significant antimutagenic and anticarcinogenic effects (Azuine et al., 2012). Silver nanoparticles synthesized using bacterial canthaxanthin pigment have shown cytotoxic effects in HaCaT cell lines (immortalized keratinocyte line) and demonstrated no side effects when tested for their wound healing capacity (Venil et al., 2021). In mammalian cells, canthaxanthin is not transformed into active retinoids, suggesting that its lipid antioxidant effects may be responsible for its inhibitory activities on conversion (Pung et al., 1988).
The antiproliferative effect of methanolic extract of Moringa oleifera leaves (MOLME) was studied in Swiss albino mice using Ehrlich ascites carcinoma cell lines. Canthaxanthin, as an important bioactive component of MOLME, has been found to inhibit cancer initiation, showing a 65% decrease in mammary cancer incidence in rats induced by dimethylbenzanthracene (Das et al., 2021). Oral administration of canthaxanthin has led to a significant reduction in tumor size and number in hamster buccal pouch carcinogenesis (Schwartz et al., 1988). Huang et al. (1992) observed a significant inhibitory action of canthaxanthin on B16F10 melanomas and PYB6 fibrosarcoma tumor cells. In Human WiDr colon adenocarcinoma and SK-MEL-2 melanoma cells, canthaxanthin induced apoptosis (Palozza et al., 1992). Canthaxanthin has also shown a decrease in the number and size of liver preneoplastic foci (induced by aflatoxin B1) in male weanling rats through the variation of aflatoxin B1 metabolism towards aflatoxin M1 and thus dissuading aflatoxin B1 to commence genotoxic action (Gradelet et al., 1998).
The reported antitumor and anticancer activities of canthaxanthin may be attributed to its radical trapping or chain-breaking processes (Bendich et al., 1989; Tanaka et al., 2012). This keto-carotenoid caused a reduction of oral carcinogenesis via suppression of cell proliferation and decreasing polyamine levels in oral mucosal tissues of F344 rats (Tanaka et al., 1995). However, lower plasma canthaxanthin concentration has been observed in females with cervical cancer (Palan et al., 1996). Canthaxanthin has been effective in preventing the initiation of dimethylbenzanthracene-induced mammary cancer in female Sprague-Dawley rats but has shown no significant effect on methyl nitrosourea-induced carcinogenesis of the same type (Grubbs et al., 1991). In MCF-7 and MDA-MB-231 human breast cancer cells, canthaxanthin has not demonstrated inhibitory effects (Chew et al., 2004). Similarly, canthaxanthin has shown no inhibitory effect on n-butyl-n-(4-hydroxybutyl) nitrosamine-induced bladder cancer (Mathews-Roth et al., 1991). A recent study showed the induction of apoptosis in DU145 prostate cancer cells by partially saturated canthaxanthin obtained from mutant fungi (Kumaresan et al., 2008).
Neuroprotective activities of canthaxanthin
Canthaxanthin has shown partial neuroprotective activity in chemically induced rat adrenal medulla cell deaths at lower concentrations (Chang et al., 2013). Among carotenoids, β-carotene and canthaxanthin have been identified as potent stimulators in the gap junction system. Gap junctions function as water-filled pores that allow the exchange of low molecular weight compounds, connecting the cytosol of neighboring cells (Stahl et al., 2005). Zhang et al. (1992) predicted this activity of canthaxanthin earlier and demonstrated that canthaxanthin upregulates the connexin43 gene, which encodes a major gap junction protein, in a dose-dependent manner.
In PC12 cells differentiated by nerve growth factor, treatment with canthaxanthin inhibited the release of TNF-α, IL-1, and IL-6, and also inhibited the activity of caspase-3 (Chan et al., 2009). However, the exact mechanisms by which canthaxanthin exerts its neuroprotective effects have not been fully elucidated (Guest et al., 2012).
Immunomodulatory activities of canthaxanthin
Canthaxanthin has been shown to exhibit immuno-modulating activities by enhancing the proliferation and activity of murine immunocompetent cells (Okai et al., 1996). In rat spleen, supplementation with canthaxanthin increased the proliferation of T and B lymphocytes (Bendich et al., 1989). Additionally, canthaxanthin treatment has been found to increase mitogen-induced lymphocyte proliferation without exhibiting provitamin activity. In human peripheral blood mononuclear cells, canthaxanthin treatment enhanced the expression of activation markers for natural killer cells and T helper cells (Chew et al., 2004).
Canthaxanthin has been established for the treatment of photosensitive disorders and tanning (Pangestuti et al., 2020). It has also been observed that canthaxanthin specifically affects the expression of heme oxygenase-1 upon UVA-related injury to human dermal fibroblasts (Camera et al., 2009).
A recent study demonstrated that canthaxanthin weakly inhibits the activities of cytochrome P450 enzymes, particularly CYP2C19 and CYP3A4/5. This suggests that dietary supplements of canthaxanthin may have minimal effects on drugs metabolized by these enzymes (Zheng et al., 2013). Furthermore, canthaxanthin has been found to increase the synthesis of cytochrome oxidases and peroxidases in murine macrophages (Pechinskii et al., 2014).
Some independent studies have shown that canthaxanthin acts as a significant inducer of liver enzymes, such as P4501A1 and 1A2, in rats. It co-induces 4NP-UGT and QR, leading to the production of xenobiotic-metabolizing enzymes (Astorg et al., 1994; Gradelet et al., 1996). However, this kind of enzyme induction is absent in lower animals (Page et al., 2002).
Effect of canthaxanthin on growth performances and digestion in marine animals
Studies have shown that the addition of carotenoids, including canthaxanthin, as nutritional supplements, can enhance the growth of marine fishes by increasing the utilization of nutrients. Niu et al. (2014) reported that the addition of carotenoids as supplements resulted in increased growth of marine fishes. In a recent study by Kalidoss et al. (2020), the addition of astaxanthin–canthaxanthin as a nutritional complement has resulted in increased growth and survival of Atlantic salmon fry and juveniles.
Furthermore, the addition of canthaxanthin and astaxanthin as dietary complements has been shown to increase the apparent digestibility coefficients (ADC) values, indicating improved utilization of protein and lipid components of food. For example, rainbow trout fed with canthaxanthin-supplemented feed (100 mg/kg) showed an ADC value of 61.4% (Kalidoss et al., 2020).
In addition to growth promotion, the effects of canthaxanthin-loaded liposomes, along with α-tocopherol, on various growth aspects and canthaxanthin accumulation in rainbow trout (Oncorhynchus mykiss) fillets were studied by Toan et al. (2021). The study demonstrated that fish administered with a diet supplemented with 1 g/kg of canthaxanthin and α-tocopherol-loaded liposomes (IC = 0.5%) showed a notable increase in final weight compared to those fed a nonsupplemented diet. This supplementation also resulted in a noticeable change in the color of salmon muscle (Toan et al., 2021).
Conclusion and future perspectives
The production of naturally occurring canthaxanthin from various living organisms such as bacteria, fungi, and algae is currently in the developmental stages, showing promising potential for commercial-scale production. Recent studies have identified several high canthaxanthin-yielding strains, including Gordonia jacobaea MV-26 (Scaife et al., 2012b), Dietzia maris NIT-D (Goswami et al., 2012), Dietzia natronolimnaea HS-1 (Nasri et al., 2010), Yarrowia lipolytica (Grewal et al., 2022), Rhodotorula mucilaginosa (Aksu et al., 2005), Coelastrella sp. (Corato et al., 2022), Dietzia maris AURCCBT01 (Abuthahir et al., 2021), and Paracoccus carotinifaciens VTP20181 (Duy et al., 2021).
However, due to the limited options available for the biological production of canthaxanthin, the market is predominantly monopolized by chemical synthesis methods (Scaife et al., 2012a, b). Nevertheless, there are promising developments in utilizing inexpensive carbon sources and external nutritional supplements to enhance the production of keto-carotenoids from biological sources (Domínguez-Bocanegra et al., 2004; Nasri Nasrabadi et al., 2010a; Nasrabadi et al., 2010b; Gharibzahedi et al., 2013b). Despite these advancements, the current yields of biopigments are not sufficient from an industrial standpoint (Nasrabadi et al., 2010b). Consequently, new approaches combining molecular biology and genetic engineering methods have been explored to enhance and improve canthaxanthin production, leading to a revolution and a new era in the medicinal, nutraceutical, feed additive, and biocolorant sectors (Gharibzahedi et al., 2013b).
Canthaxanthin plays a vital role in animals by promoting maturity and protecting tissues, both in plants and animals, against the harmful effects of oxidizing free radicals. Many countries regularly use canthaxanthin as a supplement in animal and fish feed, which has shown promising results. Additionally, there are synthetic forms of canthaxanthin available in the market, particularly useful in the food industry (Munasinghe et al., 2021).
Canthaxanthin is extensively utilized as a dietary supplement and colorant in the poultry and aquaculture industries. Beyond its pigmenting properties, canthaxanthin also holds potential health benefits, acting as a scavenger of free radicals, an antioxidant, and playing a role in gene regulation (Esatbeyoglu et al., 2017).
However, further investigation is necessary to understand the molecular targets through which canthaxanthin provides health benefits and to explore its biological functions. It is crucial to conduct more studies to uncover its biological role beyond its colorant properties. Additionally, the development of low-cost downstream processes and ensuring biological safety with regard to genetically modified microorganisms should be carefully considered for future perspectives (Jin et al., 2003; Goswami et al., 2010).
To date, the advancement of new transgenic strains of microbes has contributed to increased canthaxanthin production with enhanced environmental tolerance (Gao et al., 2020). Therefore, further research is needed to enhance the biological production of canthaxanthin and make it competitive with synthetic methods.