Introduction
Management of the anastomotic dehiscence within the upper gastrointestinal tract seems to be a significant clinical problem, resulting in a high rate of complications. The treatment strategy depends on the localization and the size of the damage as well as the amount of evacuated discharge and the patient’s general condition [1, 2]. A persistent leakage tends to be truly difficult in management as its evolution leads to the development of a fistula, which can penetrate to other organs, in both the abdominal and thoracic cavities.
Due to the heterogeneous nature of leaks, the therapy should be planned on an individual basis, with regard to the experience of the interdisciplinary team and the logistic capacity of the surgical center [3]. Firstly, the decision between revision surgery and noninvasive treatment should be considered. If an operation is not needed, external drainage followed by correction of fluid and electrolyte disturbances and protection against sepsis with appropriate antibiotics should be implemented [4, 5]. According to the Esophagectomy Complications Consensus Group (ECCG) guidelines, in type I anastomotic dehiscence defined as a microleakage, conservative treatment with effective drainage and temporary food restriction is sufficient. On the other hand, in type III, which stands for complete dehiscence, a revision procedure is necessary. Type II, the most common failure, indicates a partial rupture of the anastomosis, where endoscopic management, i.e. stent implantation or endoluminal negative pressure wound therapy (E-NPWT), represents the best solution [3–7].
Endoscopic techniques are especially beneficial in treatment of early diagnosed dehiscence that occurs immediately after the operation [3, 6]. In the case of chronic or recurring defects, some alternative methods may be implemented including tissue sealant, hemostatic clips, over-the-scope clips (OTSC) or platelet-rich plasma (PRP) [7–9].
Aim
The aim of this study is to present a noninvasive strategy for management of anastomotic leakages and fistulas after surgery in the upper gastrointestinal tract.
Material and methods
Material
A cohort of 19 patients with postoperative dehiscence of anastomosis in the upper gastrointestinal tract treated in the author’s clinic between 2015 and 2023 was retrospectively evaluated. The study group consisted of 10 males and 9 females aged 18 to 79. The most common indications for surgery were neoplastic disease and complicated peptic ulcer, respectively. Laparotomy was performed in 12 cases, whereas 7 underwent a laparoscopic procedure. A group of 9 patients was admitted from another medical center. Detailed characteristics of the study group are presented in Table I.
Table I
Characteristics of study group
Indication for treatment
Revision gastroscopy performed as early as on the second day after surgery was the decisive indication in the diagnosis of a leak. In the case of suspected leakage such as evacuation of pathological discharge, increased inflammatory markers and/or beginning of sepsis, endoscopy was preceded by a contrast computed tomography (CT) of the chest and abdomen. In patients admitted from another center and in cases of chronic dehiscence, gastroscopy was performed immediately after admission. Carbon dioxide (CO2) was routinely used for insufflation and the size of the anastomotic defect was described using fractional values, e.g. 1/2–1/4 of the total circumference of the anastomosis. The depth of a fistula (or sinus) and the diameter of the abscess were measured by comparison to biopsy forceps or to the size of the endoscope tip. When explicit assessment was not possible, water-soluble contrast detected under X-ray was administered to the fistula or sinus to show the disrupted area. Laboratory and imaging tests were used to confirm the initial diagnosis, facilitating the monitoring of the effects of treatment and allowing for detection of possible complications e.g. abscesses or fistulas.
Routinely, a dehiscence not exceeding half the anastomotic circumference and without or with limited septic complications not requiring urgent laparotomy was scheduled for minimally invasive therapy. In the case of extensive abscesses or concomitant damage to other organs, i.e. the liver, pleura, or large vessels, selection was based on local limitations and the patient’s general condition.
Methods
All patients were treated endoscopically using one of two methods: endoluminal vacuum therapy (EVT) or fully covered stent placement. The final decision depended on the size and location of the defect, septic foci as well as the operator’s preferences and experience. Stenting was strictly recommended in esophageal or duodenal leaks difficult to manage with no other existing complications. By contrast, an abscess, extensive inflammation or branching fistula favored EVT. Chronic ruptures and damage with delayed diagnosis were also treated with vacuum therapy. In addition, 12 patients had percutaneous drainage collecting the exudate from the leak outside the abdominal cavity. The principles of initial management are presented in Table II.
Table II
Clinical and endoscopic criteria determining the treatment method
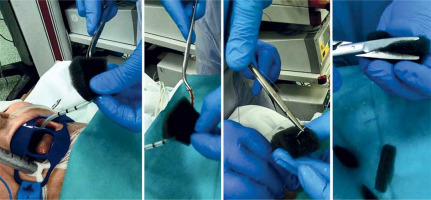
Preparing and placing the EVT dressing.
The EVT dressing was prepared manually according to the dimensions obtained during diagnostic endoscopy (Photo 1).
At the beginning a silicone drain with a diameter of 10 to 18 CH was passed through the nostrils to the oral cavity and then polyurethane foam (Renasys-F Foam Dressing Kit, Hull, UK) was fixed to it and adjusted to the size of the leakage (Photo 2).
The vacuum dressing prepared in this way was inserted under endoscopy into the dehiscence, leaving almost all of the foam in the lumen of the fistula (Photo 3).
Due to the limit of the drain diameter (not less than CH10), fistulas smaller than 7 mm were not suitable for treatment with direct EVT. In these situations an intraluminal dressing placed “en-bloc” in the area of the anastomosis was considered as an alternative (Photo 4). This technique was also used when the angle of the anastomosis to the axis of the gastrointestinal tract restricted free access of the endoscope.
In order to facilitate an application, a guidewire with a soft, hydrophilic tip (Dreamwire, Boston Scientific, Cork, Ireland) was used to place the EVT dressing into hard-to-reach damage (Photo 5).
Finally, the drain was connected to the vacuum generator (Renasys Touch or Renasys EZ Plus, Smith & Nephew Medical Ltd., Hull, UK) and usually within the first week of the therapy, a negative pressure of 90–70 mm Hg was maintained and then gradually reduced to 50–70 mm Hg. In the case of intraluminal EVT, the pressure usually did not exceed 70 mm Hg to avoid excessive damage to the mucosa of the intestinal wall. Initially, the dressing was changed after 4–5 days, and then after 2–3 days as healing progressed. The shortening of the change intervals was the result of the formation of granulation tissue and the risk of its adhesion to the foam. Moreover, to facilitate dressing replacement, the vacuum generator was turned off approximately 2 h before the procedure.
Photo 1
Endoscopic assessment of the size of the leakage in order to create a vacuum dressing. The arrow (⇨) marks the fistula channel
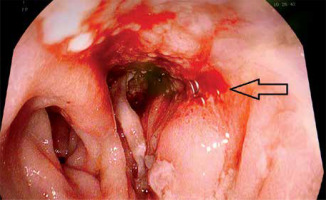
Photo 4
Intraluminal dressing as an alternative solution when it is not possible to use a direct EVT. The white arrow marks the feeding catheter, the black arrow the drain of the EVT dressing
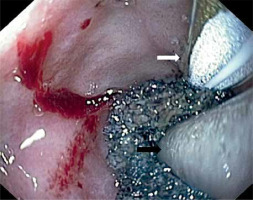
Photo 5
A guide wire with a hydrophilic tip supports the insertion of the dressing in places difficult to penetrate with the endoscope
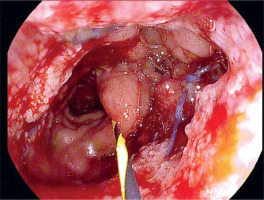
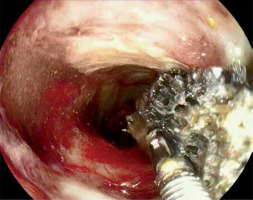
Due to the fact that narrow persistent anastomotic defects were technically unsuitable for further treatment with vacuum therapy, alternative endoscopic techniques were introduced. As long as control CT showed no abscesses or other abnormalities, attempts were made to fill fistulas with a diameter not exceeding 7 mm with tissue sealant (Tisseal, Baxter AG, Vienna, Austria). For this purpose, an ERCP catheter (ERCP Catheter SU, Endo-Flex GmbH, Voerde, Germany) was passed through the working channel of the gastroscope and inserted into the bottom of the fistula. Depending on local conditions, the area around the orifice was additionally secured with hemostatic clips (Photo 6).
Photo 6
Closure of residual fistula with fibrin glue. The ERCP catheter is used as a sealant applicator (A), then the edges of the fistula are closed with a hemostatic clip (B)
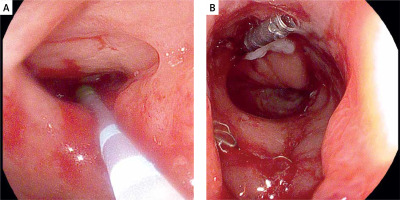
Alternatively, if the injection of the sealant could be associated with a high risk of secondary complications, the fistula was stimulated to heal with autologous platelet-rich plasma (Xerthra PRP Kit, Biovico, Poland). A dose of 1 ml of PRP with a volume of 1 ml and a platelet concentration of 106 obtained from 15 ml of venous whole blood was administered locally through an endoscopic needle. Indications for PRP administration are presented in Table III.
Stent placement
The self-expanding fully covered stent (Wall Flex Esophageal or Duodenal Stent System, Boston Scientific, Galway, Ireland) was implemented under general anesthesia. Anastomotic dehiscence was localized endoscopically and then marked under X-ray assistance. The stent was inserted with a guide wire outside and rolled out to cover the leak with a margin of undamaged wall. Additionally, the proximal cup was fixed with one or two 13 mm hemostatic clips. On the second day after the procedure, radiography of the esophagus was performed to check the position of the prosthesis.
Endoscopic surveillance
Follow-up endoscopy was performed in all patients three months after discharge. Anastomotic stricture 10 mm or less was an indication for endoscopic balloon dilation (CRE Pro Wireguided Balloon, Boston Scientific, Cork, Ireland). Routinely, during a single procedure, the stenosis was widened to 12 mm. This action was repeated after another 3 months if necessary.
Supportive treatment
Broad-spectrum individually modified antibiotic therapy was implemented depending on the result of the bacteriological examination. If an anastomotic leak was detected, oral feeding was immediately replaced with total parenteral nutrition (TPN). The metabolic rate followed the ESPEN (European Society for Clinical Nutrition and Metabolism) guidelines and Polish recommendations for clinical nutrition in oncology [10, 11].
The degree of malnutrition classified as mild, moderate or severe was determined on the basis of Nutritional Risk Screening (NRS 2002) or Subjective Global Assessment (SGA), as well as laboratory blood tests. The levels of prealbumin, albumin, complete lymphocytic count, triglycerides and total cholesterol were measured as standard on the day before the initiation of TPN, then on days 3 and 7, and on the next 7th day of the therapy. The main goal of TPN was to restore nitrogen balance and increase energy supply; therefore the daily protein intake ranged from 1.5 to 2 g/kg and calorie content 35–40 kcal/kg in relation to the ideal body weight. In the case of severe malnutrition, TPN was modified with immune modulatory amino acids (L-arginine, L-glutamine) and medium chain triglycerides to address hypoalbuminemia and lymphopenia. Parenteral nutrition was maintained throughout the duration of endoluminal therapy with the possibility of switching to the nasogastric feeding tube at a later stage of treatment. Following stent placement, oral feeding was resumed on the next day after the procedure, provided that the X-ray inspection did not indicate migration of the prosthesis.
Results
The time from surgery to the diagnosis of anastomotic leak ranged from 3 to 14 days and in 11 cases the therapy was started within the first postoperative week. The average treatment period was 27 days (14–117 days), but in the case of patients referred from outside the clinic, the beginning of treatment was delayed by 5 to 12 days.
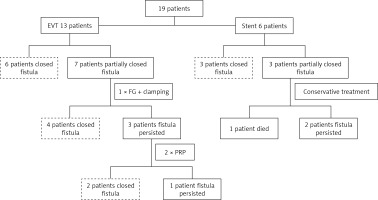
EVT was implemented in 13 cases, of which 2 cases required intraluminal ("en block") vacuum dressing. A group of 6 patients was completely healed and in another 7 cases the fistula had shrunk to a narrow sinus of less than 7 mm in diameter, in which case further treatment with EVT was impossible. Treatment was carried out by filling the defect with fibrin sealant supported in 3 patients by clamping with hemostatic clips. This enabled healing of the rupture in another 4 patients. In another three patients the residual slit diverticulum was finally closed by transmural injection of platelet-rich plasma administered in 2 sessions 14 days apart.
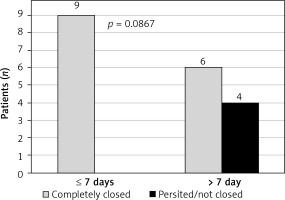
Stent placement was performed in 6 cases, of which 3 patients healed completely and the prosthesis was removed after 3 months. In the next 3 cases, a periprosthetic leak progressively occurred, leading to a permanent enterocutaneous fistula with residual evacuation of about 100 ml of intestinal contents per day. One patient died due to the development of sepsis in the course of a secondary mediastinal abscess penetrating into the left pleural cavity. A summary of the results is presented in Figure 1.
Severe malnutrition was observed in 9 patients and moderate malnutrition in 10 respectively. All patients were characterized by hypoproteinemia, hypoalbuminemia and decreased lymphocyte levels, which required TPN supported by immunogenic amino acids. In most patients (14 cases), the period of parenteral supply did not exceed 3 weeks, and in 5 patients additional enteral nutrition was provided.
Follow-up endoscopy revealed anastomotic stenosis in 5 patients treated previously with EVT. In 2 cases the endoscope did not pass through the stricture, while in the remaining cases it passed with moderate resistance. Reassessment performed 3 months after dilatation showed spontaneous resolution of the stenosis in 4 patients, while in 1 patient clinical and endoscopic progression was observed. In this case balloon re-enlargement was performed, resulting in improved patency and food passage.
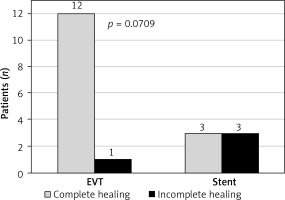
In the final assessment, the use of EVT and early initiation of treatment (up to 7 days from diagnosis) favored complete healing of the dehiscence (Figures 2, 3).
Discussion
According to the ECCG, a leakage is defined as a full-thickness GI lesion of anastomosis or conduit, regardless of the method of confirming the damage [12]. A prolonged dehiscence usually forms a fistula, i.e. a pathological connection between the lumen of the GI and the adjacent compartment such as skin, peritoneum, pleura or another organ. The presence of a rigid, epithelized canal and persistent outflow of pathological discharge are its most fundamental characteristics [13].
The high risk of leaks is favored by emergency operations, especially when the anastomosis or suturing must be provided immediately, without full preoperative preparation. The majority of these procedures are forced by perforation (neoplastic or traumatic), severe bleeding or necrosis [14–16]. In obesity the risk of dehiscence occurs as a result of difficult technical conditions as well as a temporary decrease in immunity. As the age above 70 significantly increases the risk of postoperative complications, the group of elderly patients should also be referred for surgery with special precautions [17, 18].
Complete closure of an anastomotic defect is the main goal of noninvasive treatment, but as the leakage is prolonged, this goal is difficult to achieve. Management of fistulas should rather consist of leak retardation and prevention of septic complications. The priority is the creation of effective drainage and reinstatement of oral nutrition, which improves the patient’s quality of life and prepares him, in the long term, for further revision procedures [19].
The fistulas complicated by catastrophic septic shock require an urgent redo operation. Other types of fistulas can be treated conservatively with either well-established endoscopic stenting or EVT [20]. Although stent placement allows for prompt oral feeding, it is not fully tight, and migration occurs in over 25% of cases. In addition, late complications of stenting include difficulties with its retrieval, perforation and bleeding. In addition, the stent restricts intraluminal access into the fistula, which in turn precludes any option of internal drainage [21].
Although multicenter studies indicate that EVT in a successful method resulting in fewer complications, stenting is still considered the gold standard for treatment of upper GI anastomotic leaks [22, 23]. Periodic replacement of the EVT dressing allows for constant evaluation of the healing therapy, detecting any deterioration. Moreover, replacement of the EVT dressing provides an opportunity for debridement of the fistula, which can be especially beneficial in the case of sepsis or mediastinitis. The possibility of performing a removal with percutaneous drainage is another advantage [22, 23]. On the other hand, as EVT requires relatively frequent changes, it may result in more periprocedural stress, the need for general sedation and prolonged hospitalization, so finally the overall cost of this method of treatment can be twice as high as the cost of stenting. Furthermore, standards of vacuum therapy may differ, e.g. the value of underpressure, direct or intraluminal dressing and intervals between dressing changes – all these factors are still not specified based on scientific evidence [22, 23].
Stenting seems to be more reproducible, time efficient and cost-effective. It allows earlier oral feeding, whereas intracavitary placement of EVT enables only oral fluid intake. As an alternative to surgery, it provides better quality of life and shorter hospital stay for patients [22, 23]. However, complications including periprosthetic sealing and recurrent leakage after necessary stent removal may eventually lead to surgical revision if EVT is not available [24].
The period of leaving the stent should include the time required for complete healing of the rupture and the risk of overgrowth of the graft by the surrounding granular tissue. The optimal time to remove a stent placed due to benign disorders ranges usually from 2 to 8 weeks, but in some cases this period can be extended even to 10 weeks [25–27]. The decision to terminate endoscopic treatment should take into account the results of imaging tests, the volume and type of drained discharge as well as the patient’s metabolic condition. Extensive leaks complicated by severe malnutrition require at least 3–4 weeks of sufficient sealing to reverse hypoproteinemia, anemia and weight loss. Usually after a few more weeks the defect is fully healed and the prosthesis can be safely removed [28, 29].
One of the solutions intended to facilitate the application of an endoluminal dressing is an overtube, i.e. a silicone guide resembling a sleeve-like device. The overtube stabilizes the endoscope, shortens the passage in the digestive tract and thus improves access to the working area. A modification of polyurethane sponge drainage for esophageal EVT is the ESO-Sponge (B. Braun, Melsungen AG, Melsungen, Germany), consisting of a tube inserted at the site of dehiscence and an application tool that pushes the dressing into the fistula canal. However, the relatively large diameter (13–19 mm) and wall flexibility significantly limit application within fistulas located at a significant angle to the long axis of the endoscope [30–32].
Apart from an endoscopic procedure, conservative treatment assumes metabolic assessment, prevention of systemic infection and maintenance of percutaneous drainage. In practice, the decision depends on the spontaneous reduction of discharge outside the fistula. During the period of healing the patient usually suffers from progressive malnutrition and secondary systemic complications, e.g. edema, ascites, hydrothorax and low immunity [7, 33]. Some patients may develop respiratory or renal failure and/or sepsis requiring intensive medical care. The key concept in patients with gastrointestinal fistulas is to first counteract permanent catabolism and then introduce intensive refeeding to enable proper healing. Nutritional treatment is focused on providing energy and protein as well as reinstatement of the functional efficiency of the digestive system [34, 35]. As a result, the external and internal secretion is restored, which facilitates the growth of intestinal microbiota and mobilizes the immune system. In vitro studies show that L-arginine and L-glutamine supplementation can directly influence vascular proliferation and leukocyte recruitment [10, 36].
Limitations. The results presented here are limited to a relatively small group of patients; in particular, stent implantation was performed in only a few cases. There were also differences regarding the indications for primarily procedure, the surgical approach (laparotomy vs. laparoscopy) and the timing of endoscopic treatment. The remedial management was also partly determined by the location of the anastomotic rupture. Nevertheless, the adopted strategy was coherent and the therapeutic management followed the same rules in all cases. Therefore, the conclusions reached may be applicable to a larger group of patients.
Conclusions
Management of upper GI anastomotic dehiscence using endoscopically guided noninvasive procedures seems to be effective and it allows the patient to avoid further complications. This treatment should be considered in cases with limited lesions, not exceeding half of the tract circumference without suspicion of severe sepsis. Delayed treatment beyond 7 days leads to the formation of a fistula, secondary infections and malnutrition, which significantly impedes healing and worsens the final outcomes.