Introduction
L-asparaginase (ASP), an enzyme that catalyses the hydrolysis of asparagine to aspartic acid and ammonia, is one of the basic regimen used in the treatment of acute lymphoblastic leukaemia (ALL). Neoplastic blasts have reduced expression of asparagine synthetase and therefore need asparagine from the circulating blood [1]. Asparaginase causes plasma asparagine depletion, leading to inhibition of protein biosynthesis in blast cells, cell cycle arrest, and finally cell death [2]. The efficacy of treatment is related to the duration and degree of reduction of the asparagine concentration in plasma and cerebrospinal fluid, which depends on the activity of ASP. Asparaginase activity greater than 100 IU/l is considered as therapeutic [3–6], but complete asparagine depletion was observed in some patients with lower enzyme activity [3–6]. Asparaginase can be derived from Escherichia coli (E. coli) or Erwinia chrysanthemi (Erwinia). Native and pegylated (PEG-ASP) formulations are available. They differ in pharmacokinetics, so distinct treatment schedules are used for each preparation to ensure optimal efficacy in most of the patients. Asparaginase, as a non-human protein, can cause anti-ASP antibodies generation. Hypersensitivity to ASP can be clinically visible or ‘silent’ when drug activity decreases without clinical symptoms [7–13]. The reported frequency of the presence of anti-ASP antibodies in the blood is variable and can be up to 70% [11, 12], and the frequency of allergy to ASP ranges from 30 to 75% [11–19]; it depends on the kind of formulation with the most common allergies to native E. coli ASP. Both silent inactivation and allergy are indications for switching to another ASP preparation (PEG or from another bacterial source) to ensure the efficacy of the treatment. Monitoring of the therapy with a systematic measurement of ASP activity is crucial to recognize silent inactivation [12, 17, 20–22]. Vrooman et al. [20] reported that monitoring serum ASP activity during ASP treatment can improve the outcome in paediatric ALL. The study revealed that patients with an individualized dose of ASP had 5-year event-free survival (EFS) superior to patients with fixed-dose ASP (90% vs. 82%; p = 0.04). In the Dutch Childhood Oncology Group ALL-11 protocol, an individualized dose of ASP with therapeutic drug monitoring was used, which resulted in a significant reduction in the dose of PEG-ASP with adequate levels of ASP activity and sufficient asparagine depletion [22]. However, the dose reduction with decreased levels of ASP activity did not influence the toxicity of the drug [22].
Monitoring ASP activity is also crucial for distinguishing drug-inactivating hypersensitivity and drug-induced non-inactivating reactions [22, 23]. Previously, when monitoring of ASP activity was not available, premedication with an antihistaminic prior to administration of ASP was contraindicated to avoid allergy and ASP inactivation being unrecognized. The measurement of ASP activity allows the recognition of inactivation even without clinical symptoms (silent inactivation or lack of allergy symptoms due to premedication) [22–24]. Cooper et al. found that universal premedication before administration of PEG-ASP reduced adverse events during PEG infusion, as well as the switch to Erwinase [23].
The Polish recommendations for the identification and management of clinical hypersensitivity to, and silent inactivation of, ASP preparations were published in 2016 [25] and revised in 2019 [26] following guidelines prepared by an international group of experts [27].
The aim of the study was to analyse the frequency of silent inactivation and allergic reaction and its impact on treatment results and drug toxicities in patients with ALL treated in 3 paediatric oncology and haematology departments in Poland.
Material and methods
There were 70 patients with ALL treated with ASP in the Departments of Pediatric Oncology and Hematology in Krakow, Rzeszow, and Kielce from September 2017 to December 2018, including 52 children with newly recognized ALL and 18 in the course of the therapy. All of them were enrolled in the study. There were 48 boys (69%) and 22 girls (31%), aged 1.2–17.1 years (median 5.2 years). The patients’ characteristics are presented in Table 1.
Table 1
Patients’ characteristics
| Number of patients | 70 | |
|---|---|---|
| Sex, n (%) | ||
| Male | 48 (69) | |
| Female | 22 (31) | |
| Age, years (median, range) | 5.2 (1.2–17.1) | |
| < 6 years | 41 (59) | |
| > 6 years | 29 (41) | |
| Risk groups, n (%) | ||
| Standard | 8 (11) | |
| Intermediate | 46 (66) | |
| High | 16 (23) | |
Patients were treated according to the ALL IC 2009 protocol, and in case of relapse with the IntReALL 2010 protocol. In 52 patients ASP activity was monitored from the beginning of treatment. Eighteen patients were enrolled during the course of ALL treatment (16 in reinduction, 2 in induction), in 2 of them blood samples were available and retrospectively analysed. A total of 617 measurements of ASP activity were made.
According to the ALL IC-BFM 2009 Protocol, children with ALL were classified into 3 risk groups (standard, intermediate, and high risk) depending on age at diagnosis, initial number of leukocytes, genetic abnormalities, and response to treatment [28]. There were 8 patients in the standard-risk group (SRG), 46 patients in the intermediate- risk group, and 16 in the high-risk group. The entire therapy included induction, consolidation, reinduction, and maintenance treatment. All the treatment lasted 2 years. The details of the therapy are described in the protocol [29]. In all risk groups 8 doses of native E. coli ASP (5000 U/m2 – 1 hour intravenous (IV) infusion) was given during induction, and in the high-risk (HR) group there were an additional 12 doses of ASP in early intensification. In case of allergy or silent inactivation, every 4 doses of this preparation were substituted with 1 dose of PEG-ASP (1000 U/m2 – 1-hour IV infusion) or 6 doses of Erwinase (10000 U/m2 – IV bolus) given every 2 days. L-ASP was used in consolidation only in the HR group. There were 6 HR blocks, with a high dose of L-ASP (25000 U/m2 – 2-hours IV) in each bock. It could be substituted by one dose of PEG-ASP (2500 U/m2 – 2-hours IV infusion) or 3 doses of Erwinase (10000 U/m2 – IV bolus) given every 2 days. During reinduction (Protocol II) there were 4 doses of native E. coli ASP (10000 U/m2 – 1-hour IV infusion) in all patients; in case of allergy, there was one dose of PEG-ASP (2500 U/m2 – 1-hour IV infusion) or 7 doses of Erwinase (10000 U/m2 – IV bolus) every 2 days. The asparaginase dosing in the ALL IC-BFM 2009 Protocol is presented in Table 2.
Table 2
Asparaginase dosing in acute lymphoblastic leukaemia IC-BFM 2009 Protocol
Relapsed patients were treated according to the IntReALL 2010 protocol. There were 2 risk groups according to the time of relapse (very early, early, or late) and the site of the relapse. The patients in the standard-risk group received 2 doses of PEG-ASP in induction and 7 doses of PEG-ASP in consolidation. In the HR groups, PEG-ASP was administered twice in the induction phase and 3 times in consolidation therapy. The dose of PEG-ASP in each administration according to the IntReALL 2010 Protocol was 1000 U/m2 (1-hour IV infusion).
All ASP preparations (native E. coli ASP, PEG-ASP, and Erwinase) were administered intravenously. No premedication was used.
Blood (1–1.5 ml) was collected before each consecutive dose and 3 days after the last dose of native E. coli L-ASP and Erwinase, and 7 and 14 days after administration of the high dose of ASP in HR blocks and PEG-ASP. The blood was centrifuged, and serum samples were stored at –20°C until examination. The activity of ASP was measured in real time, twice a week in the Department of Clinical Biochemistry of the Institute of Paediatrics of Krakow with the Medac asparaginase activity kit (Germany). The asparaginase activity of asparagine degrading enzymes was measured. The Medac asparaginase activity test was valid for the ASP activity of all commercially available ASP preparations. The limit of quantification was 30 U/l. Samples with activities below the limit of quantification were interpreted as having no ASP activity. Samples above the measuring range (600 U/l) were not diluted.
Silent inactivation was defined as undetectable (< 30 U/l) activity of L-ASP 3 days after native E. coli ASP, 2 days after Erwinase, and 14 days after PEG-ASP or ASP activity < 100 U/l 7 days after PEG-ASP, all preferably measured in 2 independent samples [25, 26].
During and after the administration of ASP, patients were observed for the allergic reaction. Common Terminology Criteria for Adverse Events v4.03 classification for allergic reactions and anaphylaxis were used to assess the grade of reaction. Grade 2–4 allergic reactions or anaphylaxis were indication to switch ASP preparation. Patients were also monitored for other toxicities of ASP such as abnormalities in coagulation tests and the presence of acute pancreatitis, thrombosis, or hyperglycaemia.
The early response to treatment was evaluated by flow cytometric-based minimal residual disease (FC-MRD) measured on days 15 and 33 of treatment and the achievement of complete remission on day 33. Survival rates were calculated to show the outcome of treatment. Overall survival was defined as the time from diagnosis to death from any cause or the last follow-up. Event-free survival was defined as the time from diagnosis to any event (death, relapse, failure to achieve remission) or to the date of the last follow-up. Failure to achieve remission was considered an event on day 0.
The follow-up was completed on 31 December 2021. The median observation time was 46 months.
All statistical analyses were performed with STATISTICA version 13, StatSoft Inc. Quantitative variables with non-normal distribution were compared with the Mann-Whitney U test or Kruskal-Wallis test with post hoc test (for more than 2 variables), and qualitative variables with the χ2 test and Fisher’s exact test.
Probabilities and standard errors of survival were calculated with the Kaplan-Meier method. Subgroups were compared with log-rank test.
The study was carried out according to the Declaration of Helsinki guidelines and was approved by the Ethics Committee of the Jagiellonian University (protocol code: 122.6120.247.2016, date of approval: 30/03/2017). Informed consent was obtained from all patients and guardians.
Results
The median activity of ASP 3 days after native E. coli ASP in the first part of induction was 270 U/l (range < 30 to > 600 U/l), in early intensification in the HR group it was 600U/l (range 378 to > 600 U/l), in consolidation (HR blocks) – 488 U/l (range < 30 to > 600 U/l), and in reinduction it was 505 U/l (range < 30 to > 600 U/l) (Table 3).
Table 3
Native E. coli asparaginase activity in patients treated according to acute lymphoblastic leukaemia IC-BFM 2009 Protocol
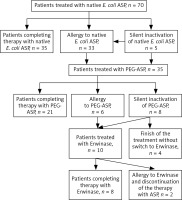
Among 70 patients treated with native E. coli ASP, silent inactivation was recognized in 5 patients (7%); in all cases it occurred during the first part of induction. All these patients continued therapy with PEG-ASP. An allergy to native E. coli ASP (at least grade 2) was found in 33 children (47%). In 8 cases the allergy occurred during induction (in 3 just after silent inactivation before the measurement result was available), in 3 children during early intensification, in 2 during HR blocks, and in 21 after the first dose of reinduction. All patients continued their treatment with PEG-ASP after allergy to native formula.
Thirty-five patients received PEG-ASP. All of them received native E. coli asp before. The median activity of ASP 7 and 14 days after PEG-ASP administration was 600 U/l (< 30 to > 600 U/l) and 79 U/l (range < 30 to > 600 U/l), respectively. In 8 patients (23%) silent inactivation was recognized (all cases during reinduction); 4 of them were not switched to Erwinase because they started the second part of reinduction before the result of ASP activity was available. In these 4 children, therapeutic activity of ASP was not ensured according to the protocol. The allergy to PEG-ASP (at least grade 2) occurred in 6 patients (17%), one during the course of induction, one during early intensification, and 4 during the course of reinduction. All of them continued therapy with Erwinase.
There were 10 patients treated with Erwinase. The median activity of ASP after Erwinase administration was 150 U/l (range < 30 to > 600 U/l). Undetectable ASP activity was found in one of the measurements but was followed by therapeutic activity after consecutive doses of Erwinase, so inactivation of ASP was not recognized. Allergy to Erwinase (at least grade 2) occurred in 2 patients (20%), in one during HR 2 block and in another during reinduction (after the fifth of 7 doses). These patients continued treatment without ASP.
The flow chart with number of patients with allergy, silent inactivation, and switch to different preparations of ASP is presented in Figure 1.
Fig. 1
Flow chart – allergy, silent inactivation, and switch to different preparations of asparaginase
ASP – asparaginase, PEG-ASP – pegylated asparaginase
The comparison of the frequency of allergy and silent inactivation between ASP preparation is shown in Table 4.
Table 4
Allergy and silent inactivation depending on the asparaginase preparation
| Parameters | Number of patients | Allergy, n (%) | Silent inactivation, n (%) |
|---|---|---|---|
| Native E. coli ASP | 70 | 33 (47) | 5 (7) |
| PEG-ASP | 35 | 6 (17) | 8 (23) |
| Erwinase | 10 | 2 (20) | 0 |
There were 6 patients who relapsed. They received second-line ALL therapy according to the IntReALL 2010 protocol. Five of them were treated with PEG-ASP, and one with Erwinase due to allergy to native ASP and PEG-ASP in the past. The median activity of ASP after PEG-ASP administration in the course of relapse therapy was 375 U/l (range: 206 to > 600 U/l). No allergy to PEG-ASP and no silent inactivation was observed. One patient was switched to Erwinase during second-line therapy due to an episode of elevated pancreatic enzyme (the criteria for pancreatitis were not fulfilled), based on the decision of the attending physician. The median activity of ASP after Erwinase administration during relapse therapy was 244 U/l (range < 30 to 464 U/l). Silent inactivation was recognized in one patient followed by allergy. The patient continued treatment without ASP.
The most common toxicities excluding allergy were abnormalities in coagulation tests. Sixty-eight patients (97%) required fibrinogen substitution. Indication for fibrinogen replacement was the level < 1 g/l. Thrombosis occurred in 2 patients (2.8%); none of them experienced silent inactivation or allergy. Hyperglycaemia requiring management with insulin was recognized in one patient (1.4%) during induction therapy. In one patient elevated amylase and lipase activity was observed (the criteria of pancreatitis were not fulfilled). The patient was switched to Erwinase based on the attending physician’s decision. There was no event of pancreatitis in the analysed group of patients.
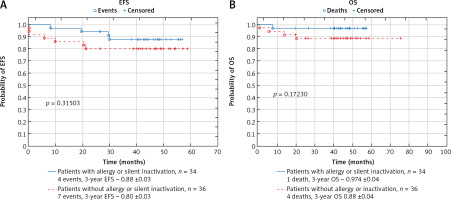
There was no significant difference in probabilities of EFS and OS between patients with and without allergy or silent inactivation (probability of 3-year EFS: 0.88 ±0.03 vs. 0.80 ±0.03, p = 0.315; 3-year OS: 0.97 ±0.04 vs. 0.88 ±0.04; p = 0.172) (Fig. 2). Among 34 patients with allergy or inactivation, 3 patients (8.8%) relapsed (3 with allergy, in one of the them silent inactivation preceded the allergy reaction, in one child silent inactivation of PEG occurred after allergy to native ASP and the patient was not switched to Erwinase), and one patient (2.8%) died of toxicities. There were 2 non-responders (5.5%), 4 relapses (11.1%), and 4 deaths (11.1%) (2 of toxicities and 2 in the course of disease progression) in the group of patients without allergy or inactivation.
Fig. 2
Comparison of survival in the group of patients with and without allergy or silent inactivation of asparaginase: event-free survival (A), overall survival (B)
EFS – event-free survival, OS – overall survival
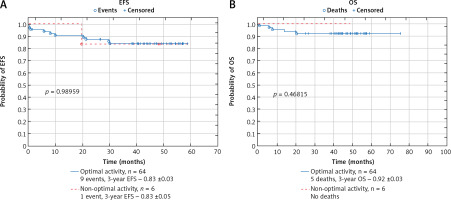
In the group of the patients who did not receive all of the planned ASP doses or substitution of inactivated ASP dose (4 patients who were not switched to Erwinase after silent inactivation of PEG-ASP and 2 patients who experienced allergy to Erwinase and continued therapy without ASP) there was one relapse (17%) and no death. The probability of EFS and OS in that group of patients did not differ significantly from patients with optimal ASP activity during the entire therapy (probability of 3-year EFS: 0.83 ±0.05 vs. 0.83 ±0.03, p = 0.989; 3-year OS: 1.0 vs. 0.92 ±0.03; p = 0.468) (Fig. 3).
Fig. 3
Comparison of survival in the group of patients with optimal asparaginase (ASP) activity and the group of patients with non-optimal ASP activity (patients who did not switch to Erwinase after inactivation of pegylated ASP or patients who continued therapy without ASP after allergy to Erwinase): event-free survival (A), overall survival (B)
EFS – event-free survival, OS – overall survival
Among 3 patients with poor response to induction therapy (no remission on day 33) median ASP activities (80 U/l, 112.5 U/l, and 142 U/l) were lower than the median value of ASP activity observed during induction therapy in the entire cohort (286.5 U/l), but they did not meet the silent inactivation criteria. All patients with silent inactivation during induction had a good early response to treatment with median FC-MRD on day 15 0.06% (range: 0–0.063%) compared to 0.7% (range: 0–93%) in the remaining patients (p = 0.097081). All of them achieved remission on day 33 with FC-MRD < 0.01% or negative. This was comparable to the group of patients who did not experience ASP inactivation in induction (median FC-MRD on day 33 0 [range: 0–37%], p = 0.257846). All patients with silent inactivation in induction were switched to PEG-ASP.
Discussion
Silent inactivation was recognized in 7% and allergy in 47% of patients treated with native E. coli ASP, which is comparable to the results reported by other authors (silent inactivation 8–29% [11, 13, 17, 20], allergy in 30–75% [11, 14, 15, 17, 19, 30]).
The switch to PEG-ASP ensured the therapeutic activity of the drug in most patients after allergy or silent inactivation of native E. coli ASP. An allergic reaction and silent inactivation of PEG-ASP occurred in 17% and 23%, respectively. Due to cross-reactivity between antibodies against native E. coli and PEG-ASP [16], the frequency of hypersensitivity reactions was higher than was described in patients who received PEG-ASP as first-line therapy [17, 30, 31]. Using PEG-ASP as first-line therapy helps to avoid cross-reactivity and reduce the frequency of allergies and silent inactivation [31].
There were 10 patients who were switched to Erwinase in our cohort. As reported by other authors [15, 17], the activity of Erwinase was therapeutic but lower than the activities of native E. coli ASP and PEG-ASP. Allergy to Erwinase occurred in 2 patients (20%); no silent inactivation was observed. In the study by Vrooman et al. [32] involving patients after allergy to native E. coli ASP, the frequency of allergy to Erwinase was 33%, while Tong et al. reported that only 3% of patients switched to Erwinase after allergy or silent inactivation of PEG-ASP experienced allergy [17].
We did not find significant differences in outcome between the groups of patients with and without allergy or silent inactivation of ASP. This can be explained by the fact that due to regular monitoring and switching to another ASP preparation after allergy or silent inactivation, therapeutic activity was ensured in almost all patients (91%). Four patients were not switched to Erwinase after inactivation of PEG-ASP in reinduction, and a further 2 patients experienced an allergy to Erwinase and continued ALL therapy without ASP. In that group of 6 patients (9% of the whole cohort) relapse occurred in one child (17%); however, the group was too small to draw general conclusions. The impact of silent inactivation or ASP allergy on outcome has been analysed by many authors before [11, 17]. The development of silent inactivation of ASP has been associated with poorer outcomes when patients were not switched to a different ASP preparation [8, 11, 17, 20]. Brigitha et al. conducted a systematic review to assess the optimal exposure to ASP needed for the best outcome in childhood ALL. They found that the level of exposure was not associated with the outcome if therapeutic levels of ASP activity levels (> 100 IU/l) were reached. The study revealed that the duration of exposure affected the outcome; however, no clear cut-off point for the optimal duration of exposure was determined [33].
The main limitation of the study is the small number of patients. Furthermore, 25% of the patients were not monitored from the start of treatment. The time of the follow-up (3 years) was also quite short. However, because median time from diagnosis of paediatric ALL to relapse reported by other authors is about 2 years [34], it seems that 3 years of follow-up was enough for preliminary analysis of survival. Further observation of the patients is planned.
Conclusions
Asparaginase is a very important component of multi-agent chemotherapy for the treatment of ALL in paediatric patients. Our study confirms the crucial role of ASP activity monitoring during ALL treatment to ensure the effectiveness of the treatment. It seems PEG-ASP should be used as the first-line preparation of ASP in the therapy of ALL in children to avoid cross-reactivity between antibodies against native E. coli and PEG-ASP and to decrease frequency of allergies and silent inactivation.








