Introduction
Gallbladder cancer (GBC) is the most aggressive biliary malignancy [1]. There is marked geographical variation in the prevalence of GBC [2-4]. The regions with the highest incidence are in South America (Chile, Bolivia, and Columbia) and the Indian sub-continent (North, East, Northeast and Central India, Pakistan, Nepal, Bangladesh, and Bhutan). The incidence in North India is 10-22/100,000 population. Regions with moderate incidence include East Asia (Korea, Japan, China) and central Europe (Slovakia, Poland, Czech Republic). Certain ethnic groups such as Hispanics, American Indians, Mexican Indians, Alaskan natives, and Asian Indians are at increased risk of GBC development [2]. Gallbladder cancer is the most common malignant cause of surgical obstructive jaundice in Northern India [3, 4]. Obesity, chronic infection (Salmonella Typhi and Helicobacter pylori), and genetic factors also predispose to GBC [5, 6]. Cholelithiasis is one of the best recognized anatomical risk factors for chronic gallbladder inflammation and GBC. It induces chronic mucosal irritation and damage which predisposes to carcinogenesis [2]. Other risk factors are gallbladder polyps, anomalous pancreaticobiliary junction, primary sclerosing cholangitis, infections (Salmonella Typhi, Salmonella Paratyphi, and parasitic infections), and exposure to various carcinogens [7, 8].
Gallbladder cancer is classified into three morphological types – mass replacing gallbladder, wall thickening, and intraluminal polypoidal lesion [9]. The ma- “jority of GBCs arise from flat dysplastic epithelium. A small percentage of GBCs (~6%) arise from a polypoidal precursor lesion [10]. A recent study reported that the relative risk of GBC was not increased in asymptomatic polyps less than 10 mm [11].
Gallbladder cancer lesions are usually solitary. Multifocal disease within the biliary tree can be due to metastasis from a single primary lesion or synchronous malignancies within the biliary tree [12, 13]. Since GBC follows the dysplasia–carcinoma in situ sequence rather than the adenoma–carcinoma sequence, two or more foci can develop malignancy in the same dysplastic environment [12]. Differentiating synchronous tumors from metastatic disease is challenging, and the criteria are not well established. The presence of multifocal disease can alter resectability and management. Thus, the reporting radiologist should actively search for multiple lesions within the biliary tree in patients with GBC.
There are no systematic imaging-based studies evaluating multifocality in GBC. Thus, we investigated multifocal lesions in a retrospective cohort of pathologically proven GBC.
Material and methods
Patients
This retrospective study was approved by the institutional ethics committee. The need for informed written consent was waived. Medical records and cross-sectional imaging (contrast-enhanced portal venous phase computed tomography [CT]/magnetic resonance imaging [MRI]) studies of clinically suspected GBC from January 2019 to December 2022 were reviewed. The medical records were analyzed for histopathological (core biopsy/post-operative specimen) or cytological (image-guided fine needle aspiration cytology [FNAC] or biliary brushing) diagnosis of GBC. Patients without a histological or cytological diagnosis, those who had undergone chemotherapy, surgery, or stenting before imaging, and those with non-contrast or suboptimal imaging were excluded. Two radiologists independently reviewed the imaging of these patients for multifocal disease.
Image acquisition
Computed tomography
CT scans were performed on one of the three multidetector row scanners (Somatom Definition Flash, Siemens Healthcare; Somatom AS, Siemens Healthcare; Philips iCT, Philips Healthcare) after intravenous injection of 80-100 ml of iodinated non-ionic contrast material (Omnipaque 300 mg, GE Healthcare) using a pressure injector at a rate of 34 ml/second. Biphasic scans were acquired during the late arterial phase (upper abdomen) and portal venous phase (whole abdomen). Bolus-tracking of contrast material was used to determine the optimal delay in acquisition for the arterial phase. The arterial phase was triggered 17 seconds after attenuation of 100 HU was achieved in the region of interest placed in the aorta at the level of the celiac axis. The venous phase was acquired at 70 seconds after the start of the contrast injection. The radiologists reviewed the portal venous phase axial and multiplanar reconstructed CT images.
Magnetic resonance imaging
All MRI scans were performed on a 1.5-T scanner (Magnetom Aera, Siemens). The following sequences were acquired: half-Fourier single-shot turbo spin-echo (HASTE) in axial and coronal planes (TR/TE = 1000/154 ms), axial T1-weighted DIXON (TR/TE = 80/2.4 and 4.8 ms), and diffusion-weighted imaging (DWI) [14]. DWI was performed in the axial plane using three b-values (b = 0, 400, and 800) (TR/TE 5800/52, image matrix, 134X134, and voxel size 1.6 × 1.6 × 5 mm). The built-in software generated the apparent diffusion coefficient (ADC) maps. Post-contrast T1-weighted volumetric interpolated breath-hold examination (TR/TE = 5/1.58 ms) was performed following an intravenous injection of 10 ml of gadoterate meglumine (Dotarem, Guerbet). Arterial, portal venous, and delayed phase images were acquired in the axial plane at 35, 65, and 180 seconds. Coronal images were also obtained in the portal venous phase. Thick and thin-slab magnetic resonance cholangiopancreatography (MRCP) images were also acquired. For this study, axial and coronal portal venous phase MRI and MRCP images were reviewed.
Image evaluation
Two radiologists (with one year of post-training experience in abdominal imaging) read the images independently, and multifocal GBCs were identified.
Multifocality was defined as the presence of two or more sites of abnormal wall thickening, intraluminal polypoidal lesions or masses in the gallbladder, cystic duct, or the extrahepatic bile ducts (common hepatic duct or common bile duct) with an intervening area of normal gallbladder/extrahepatic bile ducts.
In cases with multifocal disease, the following parameters were recorded: gallbladder stones (based on ultrasound reports), sites of involvement (fundus, body, neck), morphological subtypes of GBC at each site, size of each lesion, distance between the two lesions, involvement of adjacent liver parenchyma, biliary involvement (cause and level of obstruction), lymph nodal involvement (locoregional and distant [aortocaval, precaval, para-aortic, iliac]), vascular involvement, ascites, liver and omental/peritoneal metastasis, and resectability [1].
In the event of discrepancies between the radiologists, a radiologist with eight years of post-training experience in abdominal imaging reviewed the cases and a consensus was reached.
Results
Of the 1027 patients with suspected GBC, images of 324 cases with proven GBC were screened for multifocality. Of these, 17 (5.2%) patients (13 females, mean age 54 ±11 years) had multifocal disease. The diagnosis of GBC was based on histopathology of resected specimen in two patients, histopathology of core biopsy in four patients, FNAC in nine patients, and biliary brushing in two patients. Twelve patients had CT, and five patients had MRI. All patients had two sites of involvement. Cholelithiasis was seen in 53% of cases, with most cases showing multiple calculi (41.2%). The most common sites of multifocal disease were the fundus and neck region (58.8% cases), followed by the fundus and common bile duct (29.4%) (Fig. 1). Wall thickening type of GBC was the most common morphological type (85.3%), followed by mass forming type (14.7%). None of the cases showed the polypoidal type of GBC. Most (70.6%) patients showed similar morphology at both sites.
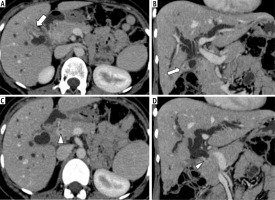
Fig. 1
Axial (A, C) and coronal reformatted (B, D) contrast-enhanced CT images of abdomen in a 39-year-old woman with histopathological diagnosis of gallbladder cancer show enhancing mural thickening in gallbladder fundus (solid white arrow) that has an indistinct interface with adjacent liver segments. There is enhancing mural thickening of the common bile duct (white arrowhead), causing upstream biliary dilatation. The confirmation of multifocal disease was based on endoscopic retrograde cholangiopancreatography-guided brush cytology from common bile duct thickening and ultrasound-guided fine needle aspiration cytology from the gallbladder lesion
The mean wall thickness in wall-thickening type of GBC was 8.58 ±4.08 mm, and the mean size (largest diameter) of mass-forming GBC was 25 ±4.69 mm. The mean distance between the two sites was 41.7 ±27.2 mm.
Adjacent liver infiltration was seen in 64.7%. Biliary involvement was seen in 94.1% (n = 16) of cases, with common sites of obstruction being the primary biliary confluence and common hepatic duct (53%), the distal common bile duct (23.5%), and the proximal/mid common bile duct (17.6%). Biliary obstruction was secondary to mural lesions in 12/16 cases. Associated regional and distant lymphadenopathy was seen in 76.5% and 17.6% of patients, respectively. Vascular involvement was seen in 47% of cases, with 35.3% showing both arterial and venous involvement, 5.9% showing only arterial, and 5.9% showing only venous involvement. Ascites was seen in 41.2% of cases. Liver and omental/peritoneal metastases were seen in 53% and 35.3% of cases, respectively. Most (70.6%) GBCs were unresectable (Table 1). Overall, near-perfect interobserver agreement (k = 0.9485) was observed for multifocality.
Table 1
Details of multifocality in 17 patients with gallbladder cancer
After a median follow-up of 8 months, 10 (58.8%) patients died. Of these, two patients received neoadjuvant chemotherapy followed by surgery, 4 patients received chemotherapy, and 4 patients received best supportive care. One patient was receiving chemotherapy at the time of writing this study. Six patients were lost to follow-up and could not be contacted.
Discussion
In this retrospective study evaluating multifocality in GBC, 5.2% of patients had multifocal disease. All patients had two lesions. Wall thickening was the most common morphology associated with multifocality followed by mass forming type.
Most patients in our study had unresectable diseases. Our study is the first systematic imaging study to report multifocality in GBC and to analyze the imaging findings in detail.
Several factors determine prognosis in GBC. The presence of jaundice or biliary obstruction in GBC is a predictor of advanced disease with a dismal prognosis [12]. Previous studies have shown that these patients have higher recurrence rates [15]. However, a few recent studies reported that biliary obstruction does not necessarily suggest inoperability, and aggressive surgery in these patients might improve their long term-survival [16, 17]. Lymph node metastasis in GBC varies from 0 to 16% in patients with T1 stage tumors. In patients with T4 stage disease, 67-80% have lymph node metastases. Studies have shown that lymph node metastasis in GBC is associated with a higher risk of recurrence and dismal prognosis [18]. Perineural invasion is common in GBC as the gallbladder and biliary tree are surrounded by a rich neural network that acts as a route for tumor spread. Previous studies have shown that perineural invasion is an adverse prognostic factor [19, 20]. Vascular spread of GBC is rare. Portal vein invasion or tumor thrombus can be seen in the advanced stage. Localized hepatic involvement near the primary lesion is more frequent in patients with vascular invasion than disseminated multiple nodules, which can occur due to the involvement of retroperitoneal veins in late-stage disease [21, 22]. Vascular resection/reconstruction would be necessary for advanced GBCs with vascular invasion, which is more challenging and associated with higher rates of complications and poor survival [23]. Gallbladder cancer also frequently involves the gastrointestinal tract (16.5% of cases), with the most common sites of involvement being the duodenum (55.8%), followed by hepatic flexure (23.3%), antropyloric region (9.3%) and transverse colon (2.3%) [24]. Though gastrointestinal involvement in GBC significantly reduces the resectability, it does not necessarily preclude resection [25].
Although multifocality in GBC also affects the prognosis and management, the data are limited [12, 13]. Multifocal disease in GBC represents either metastasis from a single primary lesion or synchronous malignancies of the biliary tree. In a previous study, synchronous gallbladder and extrahepatic bile duct tumors were reported in resected specimens in 10/190 patients [13]. Of these, seven patients had synchronous lesions, and three had metastatic lesions. Calculi were present in 4 patients. The nodular type was most common. The fundus was the most common site of origin in the gallbladder, and the middle third of the common bile duct was the most common site in the extrahepatic bile duct [13].
It is challenging to differentiate synchronous from metastatic disease. The treatment options and prognoses may differ [12]. The following criteria were suggested to differentiate synchronous tumors from metastatic spread: no direct continuity between the two tumors, growth pattern typical of a primary tumor, and clear histologic differences between the two tumors [26, 27]. However, since these criteria were inadequate to prove the synchronous origin of tumors, Kurosaki et al. advised a mapping technique to confirm the distinctness of the two lesions [13].
Exposure to the same dysplastic environment leads to chronic mucosal inflammation, causing genetic mutations such as TP53 and KRAS, and development of synchronous biliary malignancies [12, 28]. Multifocality can also be due to the intra-epithelial/intraductal spread of the tumor, which has been demonstrated pathologically in 4% of cases with papillary adenocarcinomas [21]. Multifocal disease is associated with poor prognosis due to higher incidence of unresectability (70.6% of cases in our study were unresectable). Synchronous malignancies have a better prognosis than metastatic lesions if the lesions are entirely resectable at each site [12, 13].
Multifocal disease is frequently missed on preoperative imaging [13]. Findings on pre-operative imaging in cases of GBC that must raise suspicion for multifocal disease include presence of biliary obstruction, anomalous pancreaticobiliary junction, or congenital biliary dilatation [12, 13]. Artificial intelligence and deep learning may assist radiologists in detection of multiple lesions [29]. Knowledge about multifocal GBC and careful preoperative imaging evaluation of the rest of the biliary tree in cases of GBC are essential [12, 13]. If multifocal disease is detected on pre-operative imaging, extrahepatic biliary tree resection must be considered to decrease the recurrence and improve the patients’ survival.
There were a few limitations to our study. First, despite evaluating a large cohort of patients with GBC, the number of patients with multifocal disease was small. Second, as most cases were unresectable, a detailed histopathological/cytological analysis of each lesion was unavailable. Therefore, characterization of the multifocal involvement into metastatic lesions or synchronous lesions could not be done. Third, we did not specifically evaluate the risk factors for multifocality and the impact of multifocality on the patient outcomes. Finally, we could not perform a subgroup/multivariate analysis to assess the impact of several factors on outcomes in patients with multifocal disease.
In conclusion, although multifocal disease in GBC is rare, it is essential to detect it preoperatively for appropriate patient management and to reduce the chances of recurrence of GBC.






