Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) as one of the most common chronic liver diseases is diagnosed in an alarming number of adults worldwide [1]. MASLD is characterized by increased deposition of lipid droplets in the cytoplasm of hepatocytes detected using histological evidence or radiological imaging, with the presence of at least one additional metabolic risk factor, i.e., overweight/obesity, type 2 diabetes mellitus or metabolic dysregulation [1, 2]. Increased intracellular triacylglycerol (TAG) accumulation may favor the development of a more severe form of liver injury defined by steatosis concomitant with active hepatic inflammation change, which is classified as steatohepatitis that may progress to fibrosis [1]. It is important to focus on inhibiting the development of an inflammatory state, which may significantly rate-limit the progression of simple hepatic steatosis to hepatitis. The hope of identifying an effective targeted pharmacotherapy may be found in numerous available natural compounds such as N-acetylcysteine (NAC). NAC is also considered as a well-tolerated pharmaceutical agent of plant origin without considerable side effects [3]. There are no data assessing the effect of this antioxidant in rats subjected to a high-fat diet (HFD) with attention to the development of an inflammatory state and predominantly its precursor and eicosanoid profile as a crucial factor in MASLD deterioration. In this study, we investigated the potential protective role of NAC in the liver inflammatory conditions by observing changes in the level of arachidonic acid (AA, 20:4 n-6), a precursor for inflammatory mediators, during simple steatosis development and its progression to steatohepatitis. We evaluated how NAC can prevent the occurrence of inflammation by suppressing the generation of AA derivatives, and the alteration in the cytokine profile during MASLD induced by high-fat feeding.
Material and methods
Study design
The experimental procedures were carried out on four-week-old male Wistar rats with an initial body weight of 50-70 g. The animals were housed in approved holding conditions, i.e., at a 22 ±2°C air temperature, a reverse 12/12 h light/dark cycle with unlimited access to water and chow. After one week of acclimatization, animals were randomly divided into four experimental groups (n = 10): 1) Control group – individuals receiving a regular rodent diet (kcal distribution: 65.5% carbohydrate, 24.2% protein, and 10.3% fat); 2) HFD group – individuals receiving a high-fat diet (kcal distribution: 59.8% fat, 20.1% carbohydrate, and 20.1% protein); 3) NAC group – individuals receiving a regular rodent diet with NAC; 4) HFD + NAC group – individuals receiving a high-fat diet with NAC. To rats from NAC and HFD + NAC groups, N-acetylcysteine (Sigma-Aldrich, Saint Louis, MO, USA) solution prepared in normal saline was applied intragastrically once daily via gastric gavage. Based on the currently available literature, the NAC concentration was established at a dose of 500 mg/kg of body weight with the simultaneous elimination of the toxic NAC effect [4, 5]. The amount of NAC was adjusted according to the rats’ body mass, which was measured every second day. To rats from Control and HFD groups, normal saline was applied intragastrically once daily via gastric gavage. At the end of the eight-week experiment, animals were anesthetized by intraperitoneal phenobarbital injection at a dose of 80 mg/kg of body weight. The liver tissues were promptly excised and frozen in liquid nitrogen by the use of precooled clamped aluminum tongs. The collected tissues were stored at –80°C until further measurements. The animal study was approved by the Local Ethical Committee for Animal Experiments at the Medical University of Bialystok (No. 21/2017).
Analysis of the liver histology
The liver sections of lobes from each rat were collected and immediately fixed in 10% aqueous solution of formaldehyde. The sections were dehydrated in a series of reagent grade alcohol and embedded in paraffin blocks. The thick sections were cut on a microtome, placed on slides, and stained with hematoxylin and eosin (H + E). Finally, the infiltration of inflammatory cells and lipid droplets was evaluated using a Olympus BX41 microscope with an SC50 microscope camera under 200× magnification (20× lens and 10× eyepiece) by three independent pathologists.
Analysis of the liver lipid content
Lipid fractions from the liver tissue were extracted with a solution containing chloroform/methanol at a volume/volume ratio of 2 : 1, accordingly to the Folch method [6]. To the obtained extracts, an internal standard (heptadecanoic acid, diheptadecanoic acid, and triheptadecanoic acid) was added. After that, using a heptane/isopropyl ether/acetic acid buffer (60 : 40 : 3 v/v/v), the samples were spread onto silica gel-coated glass plates (Silica Plate 60, 0.25 mm; Merck, Darmstadt, Germany) suitable for thin-layer chromatography (TLC) and separated into phospholipid (PL), triacylglycerol (TAG), diacylglycerol (DAG), and free fatty acid (FFA) fractions. Subsequently, eluents containing individual lipid fractions were transmethylated in a solution of 14% boron trifluoride-methanol and dissolved in hexane. In each lipid fraction, the individual fatty acid methyl esters (FAME) were quantified in accordance with the retention times of standards using a gas-liquid chromatography instrument (GLC; Hewlett-Packard 5890 Series II gas chromatograph; Agilent Technologies, Santa Clara, CA, USA) equipped with a capillary column and flame ionization detector (HP-INNOWax) [7]. The AA concentration in the particular lipid fractions was assessed and expressed in nanomoles per gram of tissue.
Analysis of the liver protein expression
The liver tissues were homogenized by engaging radioimmunoprecipitation assay (RIPA) buffer with the addition of phosphatase and protease inhibitors (Roche Diagnostics GmbH, Mannheim, Germany). After that, the total protein concentration was measured using a protein assay kit with bicinchoninic acid (BCA) and bovine serum albumin (BSA) as a standard. The obtained homogenates were diluted in Laemmli buffer (Bio-Rad, Hercules, CA, USA) to the same mass of protein and loaded on Criterion TGX Stain-Free Precast Gels (Bio-Rad, Hercules, CA, USA). During electrophoresis, proteins were separated and then transferred onto nitrocellulose or polyvinylidene fluoride (PVDF) membranes for wet or semi-dry conditions, respectively. The membranes were incubated in tris-buffered saline buffer with Tween-20 (TBST) and the addition of 5% BSA or 5% non-fat dry milk and immunoblotted overnight with selected primary antibodies, i.e., secretory phospholipase A2 (sPLA2; Thermo Fisher Scientific, Inc., Waltham, MA, USA), cytosolic phospholipase A2 (cPLA2; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), cyclooxygenase 1 (COX-1; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), cyclo-oxygenase 2 (COX-2; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), 5-lipoxygenase (5-LOX; Abcam, Cambridge, UK), 12/15-lipoxygenase (12/15-LOX; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), nuclear factor κ B subunit p65 (NF-κB; Cell Signaling Technology, Inc., Danvers, MA, USA), nuclear factor erythroid 2-related factor 2 (Nrf-2; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). On the second day, the membranes were incubated with TBST buffer containing horseradish peroxidase (HRP)-conjugated secondary antibodies. Protein bands were visualized by the addition of a chemiluminescent substrate (Clarity Western ECL Substrate; Bio-Rad, Hercules, CA, USA) and obtained signals were measured densitometrically with the ChemiDoc visualization system (Image Laboratory Software; Bio-Rad, Hercules, CA, USA). The expression of selected proteins was normalized to the total expression of protein, which was set as 100%.
Analysis of the liver arachidonic acid derivatives
Commercial enzyme-linked immunosorbent assay (ELISA) kits were used to assess the concentration of arachidonic acid derivatives, i.e., prostacyclin I2 (PGI2; Cusabio Technology LLC, Houston, TX, USA), prostaglandin E2 (PGE2; Cusabio Technology LLC, Houston, TX, USA), leukotriene B4 (LTB4; Cusabio Technology, Houston, TX, USA), leukotriene C4 (LTC4; Bioassay Technology Laboratory, Shanghai, China), and lipoxin A4 (LXA4; Wuhan Xinqidi Biological Technology Co., Wuhan, China).
First, 20 mg of liver tissues were homogenized in 1 ml of ice-cold phosphate buffer saline (PBS) and centrifuged following the manufacturer’s instructions. Then, the obtained supernatants were transferred into separate tubes and frozen at –80°C until appropriate determinations. The absorbances were measured spectrophotometrically at 450 nm using a microplate reader (Synergy H1 Hybrid Reader; BioTek Instruments, Winooski, VT, USA). The concentration of assessed parameters was calculated based on obtained individual standard curves. The results are expressed in nanograms per milligram of tissue for PGI2, LTC4, and LXA4 or picograms per milligram of tissue for PGE2 and LTB4.
Analysis of the liver anti-inflammatory and pro-inflammatory cytokine and chemokine profile
Briefly, the liver lysates were prepared using a cell lysis buffer (Bio-Rad, Hercules, CA, USA) with the addition of protease inhibitors factor I and II (Bio-Rad, Hercules, CA, USA) and phenylmethylsulfonyl fluoride (PMSF; Sigma Aldrich, Saint Louis. MO, USA). The lysates were centrifuged at 15,000 × g at 4°C for 10 min and obtained supernatants were transferred into separate tubes. Then, the total protein concentration was measured and set in the range of 200-900 µg/ml.
In accordance with the manufacturer’s instructions, the concentrations of the following cytokines and chemokines were measured using the Bio-Plex Pro Rat Cytokine Immunoassay Kit (Bio-Rad, Hercules, CA, USA), as a method based on multiple assay involving covalently coupled magnetic beads: granulocyte colony-stimulating factor (G-CSF), granulocytemacrophage colony-stimulating factor (GM-CSF), growth-regulated oncogenes/keratinocyte chemoattractant (GRO/KC), interleukin (IL)-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12 p70, IL-13, IL-17A, IL-18, interferon γ (IFN-γ), macrophage colony-stimulating factor (M-CSF), macrophage inflammatory protein 1α (MIP-1α), macrophage inflammatory protein 3α (MIP-3α), regulated on activation, normal T-cell expressed and secreted (RANTES), tumor necrosis factor α (TNF-α), and vascular endothelial growth factor (VEGF). First, into each well of the assay plate, a diluted couple of beads was applied. After that, blanks, standards, and samples were applied to the proper wells and incubated for one hour with shaking. Following the addition of detection antibodies, incubation, the addition of streptavidin-phycoerythrin (SA-PE) solution, and next incubation, the resuspended beads were applied. The 96-well assay plate was read on the Bio-Plex 200 System (Bio-Rad, Hercules, CA, USA) equipped with Bio-Plex Manager Software. The concentration of each cytokine and chemokine was calculated based on the standard curves for individual analytes.
Analysis of experimental data
The results from the experiment are expressed as the mean ± standard deviation (SD). The data are based on ten independent determinations in each experimental group, except for Western blot measurements in which data are based on six independent determinations. The statistical analysis was carried out using GraphPad Prism (software version: 8.2.1.; San Diego, CA, USA). The variance homogeneity and the distribution of the values were assessed by the Shapiro-Wilk test and Bartlett’s test. The statistical comparisons between experimental groups were checked by the two-way ANOVA (supported by Tukey’s test), and also the non-parametric Mann-Whitney U test or the parametric t-test for variables with non-normal or with normal distributions, respectively. For all data, statistical significance was defined as a p-value < 0.05.
Results
Influence of N-acetylcysteine supplementation on the liver histology of rats subjected to a standard or a high-fat diet
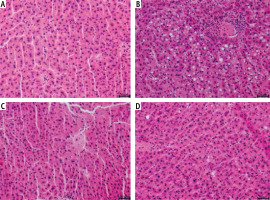
The sections of liver lobes from rats fed a standard rodent chow resulted in a normal morphological appearance (Fig. 1A). In the HFD group, all animals developed increased lipid deposition (> 5% of hepatocytes’ cytoplasm) and inflammatory cell infiltration (Fig. 1B). NAC treatment of rats fed an HFD improved balloon degeneration and decreased inflammatory cell infiltration (Fig. 1D).
Fig. 1
Histological analysis of hematoxylin and eosin stains in the following groups: A) Control, B) High-fat diet (HFD), C) N-acetylcysteine (NAC) and D) HFD + NAC in the liver tissue sections of rats subjected to a standard diet (Control) or an HFD after eight-week NAC supplementation. The data were collected from ten independent determinations of specific parts of liver lobes in each experimental group. 200× magnification. On the lower right, the calibration bar equals 50 μm
Influence of N-acetylcysteine supplementation on arachidonic acid concentration in the liver tissue of rats subjected to a standard or a high-fat diet
In the liver tissue, the administration of HFD alone and in combination with NAC caused an increase in the concentration of AA in PL and TAG (PL – HFD: +67.9%, HFD + NAC: +47.2%, p < 0.05, Fig. 2A; TAG – HFD: +410.6%, HFD + NAC: +279.0%, p < 0.05, Fig. 3B) compared to the Control group. In relation to the appropriate HFD group, arachidonic acid content was decreased in PL and TAG pools in the HFD + NAC group (PL – HFD + NAC: –12.3%, p < 0.05, Fig. 2A; TAG – HFD + NAC: –25.8%, p < 0.05, Fig. 2B). In the DAG fraction, lipid overload conditions caused an elevation in AA level (HFD: +38.2%, p < 0.05, vs. Control group, Fig. 2C), which was reduced after NAC treatment (HFD + NAC: –27.4%, p < 0.05, vs. HFD group, Fig. 2C). We also observed a decrease in the liver arachidonic acid concentration in the FFA fraction in rats that received a high-fat diet with NAC supplementation (HFD + NAC: –37.5% and –38.2%, p < 0.05, vs. Control and HFD groups, respectively, Fig. 2D). Concomitantly, in rats treated only by NAC administration, a non-significant decrease in hepatic AA content in FFA pool was observed (NAC: –16.1%, p = 0.0566, Fig. 2D).
Fig. 2
Concentration of arachidonic acid in the following lipid fractions: phospholipid (PL; A), triacylglycerol (TAG; B), diacylglycerol (DAG; C) and free fatty acid (FFA; D) in the liver tissue of rats subjected to a standard (Control) or a high-fat diet (HFD) after eight-week N-acetylcysteine (NAC) supplementation
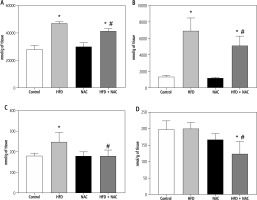
Fig. 3
Expression of proteins regulating eicosanoid synthesis, i.e., secretory phospholipase A2 (sPLA2; A), cytosolic phospholipase A2 (cPLA2; B), cyclooxygenase 1 (COX-1; C), cyclooxygenase 2 (COX-2; D), 5-lipoxygenase (5-LOX; E) and 12/15-lipoxygenase (12/15-LOX; F) in the liver tissue of rats subjected to a standard (Control) or a high-fat diet (HFD) after eight-week N-acetylcysteine (NAC) supplementation

Influence of N-acetylcysteine supplementation on the expression of proteins from eicosanoid synthesis pathway in the liver tissue of rats subjected to a standard or a high-fat diet
An HFD provoked an increase in the expression of secretory phospholipase A2 (sPLA2), cytosolic phospholipase A2 (cPLA2) and cyclooxygenase 1 (COX-1) (HFD: +14.7%, +35.0% and +23.3%, p < 0.05, respectively, Fig. 3A-C) in comparison with the Control group. Moreover, NAC application reduced the expression of cPLA2 in the lipid overload conditions (HFD + NAC: –19.6%, p < 0.05, vs. HFD group, Fig. 3B). We also observed a non-significant decrease in COX-1 expression in rats receiving an HFD with NAC supplementation (HFD + NAC: –12.4%, p = 0.0616, vs. HFD group, Fig. 3C). In the case of cyclooxygenase 2 (COX-2) expression, a decrease in the HFD group was observed (HFD: –21.2%, p < 0.05, vs. Control group, Fig. 3D). Additionally, rats treated with an HFD with NAC had a rise in COX-2 expression (HFD + NAC: +30.6% and +65.8%, p < 0.05, Fig. 3D) in relation with the Control and HFD groups, respectively. Our experiment also revealed an increase in the expression of 5-lipoxygenase (5-LOX) in rats receiving an HFD (HFD: +28.7%, HFD + NAC: +14.3%, p < 0.05, vs. Control group, Fig. 3E). We also observed an impairment in 5-LOX expression in the HFD + NAC group (HFD + NAC: –11.2%, p < 0.05, Fig. 3E) compared to the HFD group. In 12/15-lipoxygenase (12/15-LOX) expression we only observed a non-significantly higher level in the HFD + NAC group (HFD + NAC: +13.0%, p = 0.0556, Fig. 3F) than in the HFD group.
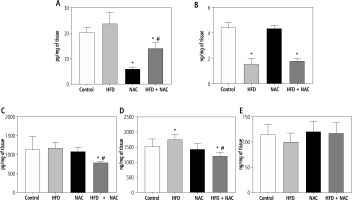
Influence of N-acetylcysteine supplementation on the concentration of arachidonic acid derivatives in the liver tissue of rats subjected to a standard or a high-fat diet
In comparison with the Control group, the concentration of prostaglandin E2 (PGE2) was diminished in the NAC and HFD + NAC groups (NAC: –70.5%, HFD + NAC: –30.5%, p < 0.05, Fig. 4A). In the HFD + NAC group, the content of PGE2 was reduced (HFD: –44.2%, p < 0.05, Fig. 4A) in relation to the HFD group. Furthermore, PGE2 level showed a non-significant increase in rats subjected to an HFD alone (HFD: +24.6%, p = 0.0959, vs. Control group, Fig. 4A). In rats received an HFD alone and in combination with NAC, prostacyclin I2 (PGI2) concentration was lower (HFD: –65.8%, HFD + NAC: –59.9%, p < 0.05, Fig. 4B) than in rats from the Control group. The administration of a high-fat diet in combination with NAC caused a reduction in leukotriene B4 (LTB4) and leukotriene C4 (LTC4) concentrations (LTB4 – HFD + NAC: –30.3% and –32.7%, p < 0.05, Fig. 4C; LTC4 – HFD + NAC: –21.4% and –31.3%, p < 0.05, Fig. 4D) compared to the Control and HFD groups, respectively. We also observed a significant increase in LTC4 content and a non-significant decrease in lipoxin A4 (LXA4) content in rats from the HFD group (LTC4 – HFD: +14.4%, p < 0.05, Fig. 4D; LXA4 – HFD: –12.3%, p = 0.0583, Fig. 4E) in relation to the Control group.
Fig. 4
Concentration of arachidonic acid derivatives, i.e., prostaglandin E2 (PGE2; A), prostacyclin I2 (PGI2; B), leukotriene B4 (LTB4; C), leukotriene (LTC4; D) and lipoxin A4 (LXA4; E) in the liver tissue of rats subjected to a standard (Control) or a high-fat diet (HFD) after eight-week N-acetylcysteine (NAC) supplementation
Influence of N-acetylcysteine supplementation on the content of selected cytokines in the liver tissue of rats subjected to a standard or a high-fat diet
The course of high-fat feeding caused increases in the hepatic levels of IL-1α, IL-7, GM-CSF, RANTES, TNF-α, VEGF (HFD: +6.6%, +11.3%, +14.4%, +28.0%, +15.7%, +4.9%, respectively, p < 0.05, vs. Control group, Table 1), and decreases in the hepatic levels of IL-1β, IL-4, IL-10, IL-13 (HFD: –2.7%, –17.0%, –12.2%, –18.9%, respectively, p < 0.05, vs. Control group, Table 1). The eight-week NAC administration to rats fed a standard diet reduced the hepatic content of IL-1α, IL-13, IL-17A (NAC: –10.4%, –18.4%, –17.9%, respectively, p < 0.05, vs. Control group, Table 1). Concomitantly, NAC treatment in HFD-fed rats caused significant increases in the hepatic concentrations of IL-1β, IL-2, GM-CSF, IFN-γ (HFD + NAC: +5.8%, +17.0%, +24.5%, +25.2%, respectively, p < 0.05, Table 1) and significant decreases in the hepatic concentrations of M-CSF and MIP-1α (HFD + NAC: –19.4%, –21.1%, respectively, p < 0.05, Table 1) compared to the Control group. Importantly, NAC supplementation to rats fed an HFD increased the hepatic content of IL-1β, IL-4, IL-5, IL-10, IL-18 (HFD + NAC: +8.7%, +23.8%, +21.4%, +23.0%, +1.7%, respectively, p < 0.05, Table 2) and decreased the hepatic content of IL-6, M-CSF, RANTES, MIP-1α, VEGF (HFD + NAC: –8.3%, –24.8%, –22.2%, –22.5%, –4.9%, respectively, p < 0.05, Table 1) compared to rats subjected to an HFD.
Table 1
Concentration of selected cytokines and chemokines in the liver tissue of rats subjected to a standard (Control) or a high-fat diet (HFD) after eight-week N-acetylcysteine (NAC) supplementation
Discussion
In this study, we focused on the potential hepatoprotective effect of N-acetylcysteine on the development of inflammation state during MASLD induced by an HFD. In the histological H + E staining, we observed increased lipid accumulation in the cytoplasm of hepatocytes, which may contribute to the development of steatosis and cause inflammation. Indeed, the infiltration of inflammatory cells in the liver tissue contributes to the deterioration of fatty liver diseases and favors liver damage by occurrence of inflammatory state [8–10]. Importantly, NAC treatment impaired the formation of lipid droplets and cell infiltration in the liver tissue of rats receiving a high-fat feeding. This is in line with the Thong-Ngam et al. study, in which NAC supplementation decreased fat deposition and hepatic inflammation [11]. In our study, we observed a rise in the hepatic level of arachidonic acid in all lipid classes. Arachidonic acid constitutes the precursor for pro-inflammatory lipid mediators, which induces and escalates the inflammatory state, thus playing an important role in MASLD deterioration [12]. Ma et al. reported that in the time-dependent model of non-alcoholic fatty liver disease (NAFLD) a high-fat diet enhanced the hepatic level of AA after 4 and 8 weeks of experimental feeding but the most dramatically increased AA was observed at week 8, indicating possible progression of NAFLD to non-alcoholic steatohepatitis (NASH) [13]. The increasing AA level is associated with enhanced phospholipase A2 activation that exacerbates the release of AA from membrane phospholipid pools, allowing its further transformation to eicosanoids. Particularly, the secretory isoform of PLA2 (sPLA2) exerts a specific function by initiating alteration in membrane phospholipid due to activation of the cytosolic phospholipase A2 (cPLA2)-mediated response [14]. We founded an increase in the expression of secretory and cytosolic phospholipases A2 in the HFD group, implying the release of AA for generation of its derivatives in rats receiving a diet rich in fat. Importantly, in the HFD + NAC group we observed a decrease in AA level in all examined lipid fractions with a significant reduction only in cPLA2 expression, corresponding to the conditions rich in fat. In the lipid overload conditions, arachidonic acid released during phospholipase A2 activation can be then metabolized into two crucial cyclooxygenase and lipoxygenase pathways. Correspondingly, the administration of HFD caused an increase in the expression of isoform 1 cyclooxygenase (COX-1), which is mainly constitutive, present in many tissues, and which is responsible for synthesis of prostaglandins such as the cytosolic form of prostaglandin E2 (PGE2) [2]. We also observed a decrease in expression of hepatocyte-specific inducible (regardless of the pro-inflammatory factors used) isoform cyclooxygenase 2 (COX-2), which has a prominent role in the regulation of growth factors and levels of several cytokines during the inflammatory state underlying the pathogenesis of metabolic disorders [2, 15]. Arachidonic acid derivatives from the COX-2 pathway such as prostacyclin I2 (PGI2) and microsomal isoform of PGE2 are involved in the progression of metabolic disorders, and the adaptation to the host immune response [2]. In our study, changes in the expression of both COX isoforms resulted in a slight increase (not significant) in PGE2 level and decrease in PGI2 level in the lipid overload conditions. The lack of significant changes in PGE2 may be due to the fact that the synthesis of microsomal and cytosolic PGE2 forms is catalyzed by COX-2 and COX-1, respectively, as already mentioned above; so, the total amount of PGE2 may not have changed. More importantly, NAC administration to rats fed an HFD caused an increase in the hepatic-specific COX-2 expression and a reduction in PGE2 level without changes in PGI2 content and COX-1 expression. Rajakrishnan et al. found that NAC supplementation reduced hepatic PGE2 concentration in rats with fatty liver induced by ethanol [16]. We suppose that NAC treatment by elevated COX-2 expression and reduced PGE2 content may exert effective preservation against liver injury. The second enzymatic AA pathway is LOX, in which two basic isoforms, i.e., 5-LOX and 12/15-LOX, can be distinguished. The products of 5-LOX pathway are pro-inflammatory family mediators, including 4-series leukotrienes, which have been proven to be associated with the pathophysiology of MASLD development and its progression to hepatitis [13]. 12/15-LOX converts free arachidonic acid via intermediate products to lipoxins. 4-series lipoxins exhibit anti-inflammatory properties and are capable of regeneration of liver capacity during fibrosis development, as mediators for later stages of inflammatory state [2, 17]. In this study, an HFD caused an increase only in 5-LOX expression. The increased 5-LOX expression resulted in an increase in leukotriene C4 (LTC4) level that plays a crucial role in acute and chronic inflammation. Interestingly, in the lipid overload conditions, NAC treatment decreased 5-LOX expression and also decreased the content of LTB4 and LTC4 as well. Studies have shown that 5-LOX reduces macrophage infiltration and hepatic inflammation in metabolic diseases. The absence of 5-LOX ameliorated liver injury in mice with sustained HFD-induced liver injury and inflammation [13, 18]. Furthermore, the reduction in leukotriene levels can decrease liver tissue damage via inhibition of mononuclear cell recruitment and chemotactic effects, limiting inflammation development [19]. In the pathogenesis of fatty liver development the accumulation of lipid and reactive oxygen species activates the transcription factor NF-κB, which is quickly released from IκB complex kinase [20]. Kuhad et al. and Tuzcu et al. reported the activation of NF-κB signal transduction in obese rats induced by an HFD, so it could then induce the generation and secretion of chemotactic and various other cytokines involved in inflammatory state [20, 21]. In the lipid overload conditions, we observed an increase in NF-κB expression, which was correlated with an increased hepatic concentration of pro-inflammatory cytokines and chemokines such as TNF-α, IL-1α, and IL-1β as well as regulated upon activation, normal T cell expressed and secreted (RANTES), deteriorating steatotic changes. As shown herein, NAC supplementation in rats that received a diet rich in fat reduced the expression of NF-κB, and, related to this, the content of pro-inflammatory cytokines and chemokines (e.g., RANTES, IL-6). We also observed an increase in the content of selected anti-inflammatory cytokines, i.e., IL-4, IL-5, and IL-10 in the HFD + NAC group. These changes may suggest a reduction in inflammatory development, protecting against MASLD deterioration.
Conclusions
The administration of NAC may be a potential agent for protecting against the occurrence of inflammation during MASLD development and its progression to steatohepatitis. Our results indicated an increase in activity of n-3 polyunsaturated fatty acid (PUFA) pathway in TAG and DAG with a simultaneous decrease in activity of n-6 PUFA in all examined lipid fractions after NAC supplementation given to rats fed an HFD. The treatment with the precursor of cysteine also reduced the hepatic content of arachidonic acid as a pro-inflammatory precursor in each lipid pool, especially in phospholipid fraction. NAC administration also abolished the expression of 5-LOX, leading to a decrease in the content of pro-inflammatory leukotriene B4 and C4. Moreover, the development of inflammatory state was weakened by NAC treatment, which was reflected in a decrease in NF-κB expression, inhibiting the synthesis of pro-inflammatory cytokines and chemokines. We suspect that N-acetylcysteine can prevent hepatic inflammation in rats with HFD-induced metabolic disorder and may limit the progression of simple steatosis to fatty liver hepatitis.






