Introduction
Hybrid coronary revascularisation (HCR) is a treatment method with the inclusion of coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI) targeting patients suffering from multivessel coronary artery disease (CAD) [1].
HCR typically integrates the minimally invasive CABG procedure, where the left internal mammary artery (LIMA) is adhered to the left anterior descending coronary artery (LAD), with PCI aimed at non-LAD vessels [2]. The aim of this approach is to combine the advantages of both surgical and percutaneous revascularisation while minimising some of their respective drawbacks [1]. There are several clinical situations, where HCR is notably recommended: history of prior CABG, inadequate or poor-quality venous conduits, non-LAD lesions amendable to PCI, or LAD lesion not amendable to PCI [3]. Essentially, this novel revascularisation method manages the survival benefits associated with the LIMA-to-LAD graft while offering a comprehensive and minimally invasive cure for coronary artery revascularisation, which includes PCI for arteries other than the LAD [2].
In the literature and clinical practice, we distinguish 3 different sequences of performing the procedure: PCI before surgery, PCI after surgery (both known as 2-stage HCR), and in the case of performing both procedures in a single approach – single-stage or simultaneous HCR [2]. The possible paths for performing the surgical revascularisation during HCR include the following: conventional on-pump and off-pump coronary artery bypass grafting, minimally invasive direct coronary artery bypass (MIDCAB), endoscopic, atraumatic coronary artery bypass grafting (EACAB), robotic-assisted CABG (RACAB), and totally endoscopic coronary artery bypass (TECAB) [1]. All the mentioned techniques aim to perform LIMA-LAD anastomosis with superior long-term patency. While the available literature focuses mainly on summarising a wide range of multicentre results, patients’ characteristics, or data comparing the results depending on the selected path of revascularisation, our review aims to provide the most detailed discussion on the need for repeat revascularisation (RR) in patients after undergoing HCR, in comparison to PCI, based on recent (2018–2023) publications.
Material and methods
The studies summarised in this review were exclusively sought in English language within the PubMed and Embase databases, as well as through manual extraction of referenced literature within the previously identified manuscripts. Throughout the search, we used the following terms: (((hybrid coronary revascularization[Title/Abstract]) OR (HCR[Title/Abstract]))) AND ((PCI[Title/Abstract]) OR (PERCUTANEOUS CORONARY INTERVENTION[Title/Abstract]) OR (repeat revascularization[Title/Abstract]) OR (REPEAT PCI[Title/Abstract]) OR (REPEAT CABG[Title/Abstract]) OR (MACCE[Title/Abstract]) OR (FOLLOW-UP[Title/Abstract]) OR (LONG-TERM[Title/Abstract]) OR (SHORT-TERM[Title/Abstract]) OR (MIDTERM[Title/Abstract]) OR (OUTCOME[Title/Abstract]) OR (OBSERVATION[Title/Abstract])), and a specific time frame from January 2018 to August 2023 was applied. We decided to summarise the results of eligible studies published in the last 5 years to ensure findings that reflect the most current evidence on the analysed topic. Furthermore, both technology (new generation drug-eluting stents, improved surgical vision devices) and approach have changed in the past 10 years, and recent results cannot be compared to outdated databases from when mainly bare-metal stents were used.
After the duplicates were removed, the inclusion of the articles was performed upon screening and applying the following eligibility criteria: the study participants were patients undergoing hybrid coronary revascularisation; the control interventions consisted of PCI alone; primary or secondary endpoints in the selected studies had to include data reporting RR, regardless of the follow-up time.
Exclusion criteria included insufficient detail concerning RR and/or target vessel revascularisation (TVR) and lack of accessibility. Systematic reviews and metanalyses from previous years were also excluded from the study.
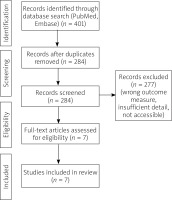
A flow diagram illustrating the records qualification process for the review is presented in Figure 1.
Data collected from the studies eligible for the review included general information about the publications (title, first author, country, DOI) as well as further details, i.e. the number of patients, inclusion criteria, interventions’ techniques, RR and/or TVR rates depending on the primary procedure, and follow-up data. Two authors independently conducted the literature search, decided on inclusion of the eligible articles, and addressed any differences and quality considerations by collaborative discussion and following inclusion or exclusion.
Results
Study selection
The initial search strategy, based on carefully selected terminology, as outlined in the Methods section, resulted in a total of 401 records, available through advanced search in the PubMed and Embase databases. After removing duplicates and following a selection process guided by specific eligibility criteria, 7 records (6 original studies [4–10], 1 follow-up research letter [6]) conducted between the years 2018 and 2023 were selected for the final analysis.
Characteristics
Our study involves a total of 28,672 patients, including 704 (2.46%) patients in the HCR group and 27,968 (97.54%) subjects in the PCI group. Because our primary objective was to consolidate the data regarding RR and/or TVR, we have provided a summary of RR and/or TVR, depending on the outcome measure reported in each of the records. Characteristics of eligible studies presented in Tables I and II, besides RR and/or TVR rates, include study type, time of patients’ enrolment, outcome measures other than RR and/or TVR (MACCE, death, myocardial infarction, stroke, all-cause mortality) with median follow-up time and HCR approach and sequence (simultaneous or 2-stage HCR). The geographic distribution of studies encompassed North America, Europe, and Asia.
Table I
Summary of the results of studies assessing repeat revascularisation in hybrid coronary interventions vs. percutaneous coronary intervention
| Ref; | First author, publication year, country | Patient enrolment | Study type | RR and/or TVR for HCR | RR and/or TVR for PCI | Other |
|---|---|---|---|---|---|---|
| [4] | Hannan E, 2021, USA | 2010 –2016 | Retrospective observation | Time [years] (No RR, No. at risk [n]; N = 335): 1 (330) 2 (272) 3 (215) 4 (158) 5 (117) 6 (80) | Time [years] (No RR, No. at risk [n]; N = 27557): 1 (23388) 2 (18442) 3 (14314) 4 (10882) 5 (7773) 6 (5243) | >RR – any unstaged revascularisation (PCI or CABG surgery) in the LAD artery; 335 HCR: 320 off-pump surgery, 5 off-pump surgery followed by on-pump surgery, 10 on-pump surgery; Interaction between RR and 6 highest volume HCR hospitals (aHR = 0.42, 95% CI: 0.26–0.69, p-value = 0.01). Examination of pre-selected subgroups of patients indicates that no patient subgroups had significant interactions between revascularisation strategy and RR in the LAD artery. |
| Freedom from RR in the LAD artery (median 4-year follow-up) HCR 91.13% vs. PCI 83.59% p-value 0.001, aHR = 0.51, 95% CI: 0.34–0.77 | ||||||
| [5, 6] | Ganyukov V, 2020, Poland and Russia [5] Ganyukov V, 2021, Russia [6] | 2012 | Randomised controlled trial | 30 days: 1.9% (1) (N = 49); 12 months: clinically driven TVR 1.9% (1); Angiography – driven TVR 11.5% (6); total TVR 13.5% (7) [6] F/u time [months]: 52.5 (min. 36) Clinically driven TVR: 16.6 (8) | 30 days: 0% (0) (N = 51); 12 months: clinically driven TVR 5.7% (3); Angiography – driven TVR 11.3% (6); total TVR 17.0% (9) [6] F/u time [months]: 52.5 (min. 36) Clinically driven TVR: 20.0 (10) | >HCR: incomplete TLR (per patient) 7.7% (4) Incomplete TLR (per total number target lesions in study group) 2.7% (4/149); PCI: incomplete TLR (per patient) 5.7% (3) Incomplete TLR (per total number target lesions in study group) 2.1% (3/146). |
| p-value NS p-value NS [6] | ||||||
| [7] | Basman C, 2020, USA | 2009–2016 | Retrospective observation | Time [years] (Freedom from RR, No. at risk [n]): 2 (69) 4 (30) 6 (17) 8 (0) (8-year f/u) RR: 16 TVR: 6 (3 problems with the LIMA to LAD graft, 3 in non-LAD vessels), 10 (62.5%) de novo lesions | Time [years] (Freedom from RR, No. at risk [n]): 2 (63) 4 (37) 6 (18) 8 (0) (8-year f/u) RR: 18 TVR: 10 | >TVR – a repeat intervention for a prior stented lesion, either within the stent itself or within 5 mm of the stent, and/or a repeat procedure for a lesion that was previously surgically bypassed. In the TVD patient population with intermediate SYNTAX scores, although midterm survival is comparable across treatment arms, morbidity may be higher after PCI, particularly with respect to the increased incidence of RR and new MI. |
| RR p-value NS TVR p-value NS | ||||||
| [8] | Modrau IS, 2020, Denmark | 2010–2012 | Retrospective observation | (3-year f/u) 21.4% (index hospitalisation (8/16), prescheduled 1-year angiography (8/16); 9/16 (56%) during the first year were driven by angiographic findings w/o associated symptoms of ischaemia) Time [years] (Freedom from RR, No. at risk [n]): 1 (85) 2 (76) 3 (75) | (3-year f/u) 12.6% Time [years] (Freedom from RR, No. at risk [n]): 1 (93) 2 (89) 3 (87) | >Multivessel PCI was performed “one-stop” in 75 (73%) patients and staged in 28 (27%) patients. HMR was converted to CABG in 3 patients and censored as RR and analysed as intention to treat (failed PCI for total chronic occlusion in 2 patients, LIMA graft thrombosis and procedure-related myocardial infarction in 1 patient). |
| p-value NS | ||||||
| [9] | Qiu J, 2019, China | 2009–2016 | Retrospective observation | TVR Perioperative: 0/52, Mid-term 59 months (interquartile range, 42 to 79 months): 2/44 (4.5%) | TVR Perioperative: 0/44, Mid-term 59 months (interquartile range: 42 to 79 months): 10/45 (22.2%) | - |
| p-value 0.015 | ||||||
| [10] | Repossini A, 2018, Italy | 2013–2016 | Retrospective observation | (100% complete angiographic follow-up at 12 months); TVR (4): 2 in-stent restenoses of left main-Cx stent, 1 poststent stenosis, 1 in-stent restenosis on RCA lesion; no procedures on LAD for LIMA-LAD graft failure or stenotic anastomosis | 7 patients: plain old balloon angioplasty (POBA; kissing balloon) on left main for bifurcation initial restenosis; 3 in-stent restenoses treated by new PCI, 4 poststent stenosis, 2 incomplete distal stent expansion, 8 in-stent restenosis on a RCA lesion | >HCR vs. PCI studies demonstrated that the complexity of the coronary lesion directly affects the outcomes of PCI, especially the TVR (mainly concentrated in the LAD), whereas PCI with DES for non-LAD offered low and similar TVR rates in both HCR and PCI groups. PCI stenting on left main was an independent predictor of MACCEs (hazard ratio 4.1, 95% CI 2.4–11.3; p-value 0.001) and TVR (hazard ratio 3.9, 95% CI 1.36–9.64; p-value 0.002). Female sex was an independent predictor of TVR (hazard ratio 2.1, 95% CI: 1.12–4.65; p-value 0.049). |
| Time [months] – Survival freedom from TVR (HCR/PCI) [n]: 3 – 67/102, 6 – 61/97, 9 – 54/90, 12 – 43/81, 15 - 41/64, 18 – 40/57; Survival freedom from TVR: (HCR: 93.3 (4.6)% PCI: 75.5 (5.6) % | ||||||
| p-value 0.002 | ||||||
[i] aHR – adjusted hazard ratio, CABG – coronary artery bypass grafting, CI – confidence interval, DES – drug-eluting stents, HCR – hybrid coronary revascularisation, HR – hazard ratio, LAD – left anterior descending artery, LIMA – left internal mammal artery, MACCE – major adverse cerebral and cardiac events, MI – myocardial infarction, PCI – percutaneous coronary intervention, POBA – plain old balloon angioplasty, RCA – right coronary artery, RR – repeat revascularization, TLR – target lesion revascularisation, TVD – triple vessel disease, TVR – target vessel revascularisation.
Table II
Additional data regarding characteristics of patients in included studies
| Ref | First author, publication year, country | Eligibility criteria | Number of patients HCR/PCI | HCR approach | Simultaneous HCR | HCR: PCI after surgery | HCR: PCI before surgery | Other outcomes HCR/PCI |
|---|---|---|---|---|---|---|---|---|
| [4] | Hannan E, 2021, USA | 1) MV-D (≥ 70% stenosis in ≥ 2 major epicardial CA), including diseased LAD artery (≥ 70% stenosis) 2) Minimally invasive CABG surgery (no sternotomy) or PCI in the LAD artery 3) Elective PCI in one or more other diseased arteries within 60 days before or after the LAD procedure without any other concomitant major cardiac surgery | 335 (1.20%) /27,557 (98.80%) | Isolated CABG surgery performed on the LAD artery with minimally invasive surgery (off-pump/on-pump) + PCI procedures performed within 60 days before or after the CABG surgery in non-LAD vessels | 44% | 18% | 38% | Median f/u time [years]: 3.81 (HCR), 4.20 (PCI) [Prevalence HCR/PCI; Four-year mortality: hazard ratio (95% CI) of HCR/PCI, p-value] MI within 20 days 28.96%/37.53%; 0.76 (0.44–1.32), 0.44 Cerebrovascular disease 10.15%/8.63%; 1.14 (0.62–2.10), 0.37 BMI < 25 kg/m2 27.76%/21.67%; 0.63 (0.36–1.12), 0.003 |
| [5, 6] | Ganyukov V, 2020, Poland and Russia [5] Ganyukov V, 2021, Russia [6] | 1) Angiography-confirmed MV-CAD involving LAD and a significant (≥ 70% diameter stenosis, DS, on quantitative coronary angiography, QCA) lesion in at least one major non-LAD epicardial vessel of ≥ 2.5 mm in diameter, amenable to PCI and CABG and HCR 2) Lesions of 50–70% DS were subjected to functional evaluation and were considered the study target lesions (i.e, were labelled for revascularization) if lesion-related myocardial ischemia was present on functional testing (fractional flow reserve, FFR, or SPECT stress imaging | 52/ 53 [6] 3-year F/u (randomized) 48 (52)/50 (53) | MIDCAB LIMA-LAD + PCI for non-LAD vessel/s | – | HCR patients, except 5 (9.8%) who required conversion to CABG had per-protocol PCI within 3 days (in most cases at 24–48 h) after performing MIDCAB LIMA-LAD anastomosis that was always the first stage of HCR | – | MACCE (death/stroke/MI/clinically driven repeat revascularisation) F/u time [days]: 30 Death 1.9% (1)/0% (0) Stroke 1.9% (1)/0% (0) MI 5.8% (3)/3.8% (2) F/u time [months]: 12 Death 5.8% (3)/3.8% (2) Stroke 3.8% (2)/0% (0) MI 5.8% (3)/7.5% (4) [6] F/u time [months]: 52.5 (min. 36) All-cause mortality 6.3 (3)/6.0 (3) MI 6.3 (3)/12.0 (6) Stroke 4.2 (2)/8.0 (4) |
| [7] | Basman C, 2020, USA | 1) Stable TVD (w/o concomitant non-coronary procedure, previous coronary and/or valve surgery, emergency/salvage surgery, hemodynamic instability) | 100 (after propensity match)/100 (after propensity match) | Off-pump robotic-assisted LIMA to LAD bypass (MIDCAB component of HCR) + PCI standard techniques (~50% radial approach); either second-or third-generation DES | 0 | 72 (MIDCAB-first approach, followed by interval PCI, typically within 4 to 6 weeks of surgery) | 28 (coronary syndrome in which the culprit lesion was deemed to be within one of the non-LAD vessels, or angiographic severity and clinical import of at least one of the non-LAD stenosis greater than that of the disease within the LAD itself. For these patients, subsequent LIMA to LAD grafting was undertaken on uninterrupted DAPT) | F/u time [days]: 30 30-d mortality 0/0 Stroke 0/0 Periprocedural MI 0/0 New-onset renal failure 0/0 Length of stay, days, mean ± SD 5.7 ±7.5/2.0 ±2.2 (p < 0.0001) Residual SYNTAX score, mean ± SD 4.5 ±4.4/ 7.1 ±6.5 (p < 0.001) Mean (SD) f/u [years]: 7.14 (0.12) Mortality 5/9 (p = 0.41) Myocardial infarction 4/5 (p = 1.0) MACE (death, repeat revascularisation, and myocardial infarction) 21/25 (p = 0.61) |
| [8] | Modrau IS, 2020, Denmark | 1) Age: 18 years 2) MVD involving the LAD | 103/103 | Offpump anastomosis of the LIMA to the LAD through a left inferior J-hemisternotomy (JOPCAB) | – | 11 (11%) | 92 (89%) | F/u time [years]: 3 MACCE (all-cause death, myocardial infarction, stroke, and repeat revascularisation at 3-year follow-up) Death 6.8%/5.8% Myocardial infarction 3.9%/3.9% Stroke 3.9%/2.9% |
| [9] | Qiu J, 2019, China | 1) The patient underwent HCR, isolated OPCAB or isolated PCI 2) The patient had two-vessel CAD including proximal LAD stenosis 3) LIMA-to-LAD anastomosis was performed in patients underwent HCR or OPCAB 4) The stents used in HCR or PCI were drug eluting stents (DES) 5) Exclusion criteria: the operation was emergent; the patient had undergone coronary revascularisation before | 47 (after propensity score matching)/47 (after propensity score matching) | LIMA-to-LAD anastomosis; the stents used in HCR or PCI were drug-eluting stents (DES) | – | – | – | MACCE (death, MI, stroke, TVR) F/u time [days]: 30 Death 0/0 MI 0/1 (p = 0.365) Stroke 0/0 F/u time [months]: 59 [42–79] Death 1/2 (p = 0.811) MI 1/3 (p = 0.411) Stroke 2/3 (p = 0.874) |
| [10] | Repossini A, 2018, Italy | 1) Critical left main stenosis or equivalent left main lesion, with or without multivessel coronary lesions 2) Primary/rescue PCI for acute coronary syndrome on non-LAD lesions with residual lesions on left main (excluded: distal heavy calcified lesions and isolated ostial or proximal-mid-body left main disease, concomitant surgical procedures in addition to myocardial revascularisation) | 67 (preoperative matched)/108 (preoperative matched) | LIMA-LAD and PCI on other target vessels (MIDCAB was performed as the first step of the hybrid revascularisation strategy, followed by PCI stenting of circumflex artery and non-LAD lesions) | 0 | 62 (unprotected LMCD, a surgical revascularization via MIDCAB was performed as the first step of the hybrid revascularization strategy, followed by PCI stenting of circumflex artery and non-LAD lesions) (a timeframe of about 1–4 weeks) | 5 (left main equivalent lesions with ostial stenosis of both LAD and circumflex artery (Cx), PCI stenting from Cx to left main was performed before MIDCAB) | MACCEs (cardiac death, stroke, AMI, repeated TVR) F/u time [days]: 30 In-hospital mortality 0/3 (2.7) (p = 0.603) Stroke 0/1 (0.9) (p = 0.839) Myocardial infarction 0/1 (0.9) (p = 0.839) Postoperative atrial fibrillation 8 (11.9)/1 (0.9) (p = 0.008) Pericardial effusion 3 (4.4)/5 (4.6) (p = 0.984) Mean (SD) f/u [months]: HCR 15.4 (2.6)/PCI 15.2 (2.8) Mortality at 18 months’ 0/0 Major cerebral adverse events 0/2 AMIs 0/7 Survival freedom from MACCEs at 12 months’ 97.2 ±2.5%/86.3 ±3.2 Survival freedom from MACCEs at 18 months’ 93.3 ±4.6%/ 72.3 ±6.3 (p = 0.001) |
[i] AMI – acute myocardial infarction, BMI – body mass index, CABG – coronary artery bypass grafting, CA – coronary arteries, DAPT – dual antiplatelet therapy, DES – drug eluting stents, FFR – fractional flow reserve, HCR – hybrid coronary revascularization, LAD – left anterior descending artery, LIMA – left internal mammal artery, LMCD – left main coronary artery disease, MACCE – major adverse cerebral and cardiac events, MACE – major adverse cardiac events, MI – myocardial infarction, MIDCAB – minimally invasive direct coronary angiography, MVD – multivessel disease, PCI – percutaneous coronary intervention, TVD – triple vessel disease.
Repeat revascularisation and/or follow-up target vessel revascularisation
In 3 out of 7 records, the RR and/or follow-up TVR rates were more favourable for HCR compared to PCI. It was observed that RR was significantly less frequently required in the case of HCR than in PCI. Hannan et al. observed after a follow-up period of 4 years freedom from RR in the LAD artery in 91.13% in the HCR group, whereas in the PCI group the rate was 83.59% with a p-value of 0.001 (aHR = 0.51, 95% CI: 0.34−0.77) [4]. Moreover, they reported an interaction between RR and the 6 highest-volume HCR hospitals (aHR = 0.42, 95% CI: 0.26−0.69, p = 0.01). An analysis of pre-selected patient subgroups revealed that there were no significant interactions between the patient subgroups and the revascularisation strategy or risk reduction in the LAD artery [4].
In a study from 2019, the perioperative TVR rate was 0 for both groups, whereas after 59 months in a mid-term follow-up TVR was performed in 2/44 (4.5%) for the HCR group and 10/45 (22.2%) in the PCI group with p-value at 0.015 [9].
In 2018, Repossini et al. reported a survival freedom from TVR at 93.3% (4.6%) for HCR and 75.5% (5.6%) for PCI (p = 0.002). According to their analysis, HCR vs. PCI trials showed that the intricacy of the coronary lesion strongly influences PCI results, particularly the TVR (which is mostly concentrated in the LAD). In contrast, PCI with DES for non-LAD provided low and comparable TVR rates in both HCR and PCI groups. Also, they observed female sex as an independent predictor of TVR (HR = 2.1, 95% CI: 1.12–4.65; p = 0.049) [10].
In the remaining 4 studies, no significant differences in RR and/or TVR rates between the 2 treatment strategies were observed [5–8]. A summary of the results is presented in Table I. Additional data regarding characteristics of the patients in the included studies are presented in Table II.
Quality considerations
In a study by Hannan et al., the issue of selection bias caused by lack of randomisation was minimised by employment of Cox proportional hazards models so as to control for differences in patient risk factors among patients undergoing the analysed procedures. Given that the study included only patients who survived long enough to have the second treatment, it was noted that for 2-stage procedures, survival bias could be present. Another constraint is that HCR encompasses a wide range of procedures, and we could not evaluate the effects of the pharmacological therapies administered between and after the procedures or assess how their usage impacted the outcomes [4].
Due to variations in SYNTAX scores, Basman et al. stated in their study that their data is not appropriate for drawing conclusions regarding the superiority of one revascularisation technique over another [7]. The Repossini et al. study did not fully document the need for clinical versus angiographic reasons for RR, which means that it is not possible to completely rule out the possibility of an excessively high rate of prudential RR in the event of initial restenosis [10].
In the HREVS RCT study the issue of patient’s choice towards a less invasive procedure was considered with result of 1 in 4 refusal rates to random treatment allocation due to preference of PCI. However, the overall recruitment rate was over 75% [5]. It was also observed that while considering the applicability of the results, the moderate angiographic complexity of multi-vessel disease (MVD) must be taken into account. This complexity reflects the necessity for the technical feasibility of HCR and multi-vessel PCI (MVPCI). Due to this criterion, cases involving left main coronary artery stenosis not amenable to HCR, severely calcified lesions, complex bifurcations, or chronic total occlusion were excluded (all of which may favour surgical interventions) [5].
The principal constraint of the Modrau study was its sufficient statistical power to draw definitive conclusions regarding MACCE endpoints. To derive meaningful insights, the results should be considered alongside other studies that contribute to the broader evidence on the topic. It is worth noticing that the retrospective SYNTAX calculation was conducted retrospectively and, therefore, did not serve as a matching criterion for control patients undergoing PCI or CABG. The notably high repeat revascularisation rate observed in the hybrid revascularisation (HMR) group was primarily a result of angiography following the protocol rather than symptom-driven, introducing a bias against HMR, because this protocol was not applied to the CABG and PCI groups [8].
Discussion
In cases where both the LAD artery and at least one other significant coronary artery are involved, HCR is an infrequent treatment path as an alternative to PCI for CAD patients [4]. In 2021 Hannan et al. reported that, because no significant difference in mortality in a median follow-up of 4 years was noted, HCR exhibits a lower rate of RR as opposed to PCI (91.13% vs. 83.59%, p = 0.001, aHR = 0.51 [95% CI: 0.34−0.77]), which creates a field for analysing whether and why it is worth to consider HCR as an alternative treatment pathway. Especially, since there is a limited number of publications regarding this criterium [4].
Several research articles have examined diverse outcomes of HCR compared to traditional PCI. In patients with MVD, both HCR and PCI showed similar 6-year risk-adjusted survival, whereas HCR patients were less likely to require a repeat LAD revascularisation at that time [4]. Eight-year survival outcomes in patients with TVD treated with HCR compared with multivessel PCI showed similar mortality rates, with HCR having a lower residual SYNTAX score [7]. In a different study, after a 3-year follow-up period, HCR has shown similar results to PCI in terms of all-cause mortality, myocardial infarction, stroke, TVR, and major adverse cardiac and cerebrovascular events (MACCE). To fully ascertain the potential of HCR as a coronary revascularisation technique in patients with MV-CAD, the study highlights the necessity for more extensive studies and longer follow-up [6].
In a study by Repossini et al., following an 18-month period, HCR demonstrated a markedly reduced incidence of MACCEs, mostly as a result of greater independence from TVR. The improved results in terms of MACCEs may be explained by the advantages of the left internal mammary artery to left anterior descending artery (LIMA-LAD) bypass versus PCI in terms of patency rates, according to the research. It also draws attention to the sequential staged method used in HCR, highlighting its possible benefits in lowering the risk of bleeding and thrombotic events in comparison to single-step revascularisation techniques [10].
HCR is gaining popularity as an alternative to CABG and PCI in the treatment of the left main artery, providing a solution that integrates the advantages of both techniques while minimising surgical trauma and postoperative complications [4, 10]. HCR is a safe procedure, providing promising midterm results, also in patients with high risk, and long-term in patients with multivessel coronary artery disease, especially in patients with proximal LAD stenosis [9, 11, 12]. The benefits of HCR are numerous; however, the decision to choose this treatment path over PCI must be made on an individual basis, taking into account factors such as lesion complexity and risk for the patient.
It must be remembered that the hybrid revascularisation protocol is not unified, and many divergences can be observed in different institutions. Those may include not only alterations in procedure sequence (PCI-first; surgery-first; one-stage), but also timing of divided procedures (days/months) and qualification criteria. Obviously, institutional experience in such procedures also contributes to the results. As such, significant variations in study results can be observed.
Clinical guidelines emphasise the limited evidence from randomised control trials to support hybrid revascularisation [13]. As such, it is essential to conduct such multicentre studies with a unified protocol, including antiplatelet treatment, and patients’ eligibility criteria. This would produce the data that would finally result in modification of European Associations guidelines. It is also essential to properly address the patient groups, because hybrid coronary revascularisation may be particularly beneficial in both young, active patients with relatively low risk of complications and in patients for whom standard surgical treatment is of great risk of complications. As such, a clear and effective protocol for such study is required.
Study limitations
The analysis has following limitations: Firstly, hybrid revascularisation protocols are different in various institutions, which leads to differences in the results. Secondly, the devices used for both percutaneous and surgical stage of hybrid revascularisation are different in different institutions, as is clinical experience. Qualification criteria vary in those facilities, suggesting that different groups of patients are included in the selected studies. Finally, mainly retrospective studies were included, which increases the risk of bias.
Conclusions
HCR as a treatment method exhibits a significant advantage over PCI in decreasing the necessity for RR and/or follow-up TVR in a subset of cases. Nevertheless, it is important to remember that in the remaining 4 out of 7 studies, no statistically significant differences were reported between the 2 therapeutic methods, emphasising the complexity and variability of outcomes associated with these therapeutic techniques.