Introduction
Cigarette smoking is an important health threat and a risk factor for the development of many human disorders, including pulmonary, reproductive, endocrine, gastrointestinal, and cardiovascular diseases and malignancies [1, 2]. Nicotine, as a major component of cigarette smoke, has physiological and pathological effects on people who use it [3]. Nicotine enters the body through cigarette smoke and, to a lesser extent, through some foods and through the skin, lungs, nasal mucosa, and mouth, and after entering the bloodstream, it spreads throughout the body [4, 5]. This component affects many physiological systems and disrupts their structure and function [6].
Liver fibrosis is one of the most important complications of liver diseases in which the structure of the liver is disrupted due to the accumulation of fibrous tissue [7]. Viral, autoimmune, hereditary, metabolic, and toxin diseases are among the factors that cause damage to the liver. Liver fibrosis and its final stage, which is known as cirrhosis, causes death and disruption in the lives of thousands of people every year [7]. Liver disease accounts for 2 million deaths and is responsible for about four percent of all deaths [8]. Deaths are largely attributable to complications of cirrhosis and liver cancer [9]. According to an estimate made in the United States, cirrhosis causes 700,000 hospitalizations every year, and therefore imposes high costs on the healthcare system [10]. As a result of fibrosis, the liver tissue hardens and the movement of blood becomes difficult in it, which is closely related to portal hypertension [11]. The growing prevalence of this disease in recent years and the burden of the disease led researchers to pay special attention to research on liver fibrosis. Many studies have investigated the effects of nicotine on the liver and epidemiological evidence has revealed that smoking is a risk factor for the progression of liver diseases [12].
Nicotine binds to nicotinic acetylcholine receptors (nAChRs) and high consumption of nicotine changes the activity of these receptors [13]. Various studies have shown that smoking leads to changes in the expression of various subtypes of nAChRs [14]. Nicotine, as an alkaloid compound present in cigarette smoke, is important in the progression of different diseases by binding to nicotinic receptors and activating signaling pathways dependent on these receptors [15, 16]. Various subtypes of nicotinic receptors may play a role in the processes of liver injury [17–19]. Watson et al. reported that α4nAChR was the most abundantly expressed subtype of nicotinic receptor in mouse liver [20]. In this study using knockout (α4 KO) mice, the processes of alcohol-induced liver damage were either absent or significantly reduced in α4 KO animals [20]. Therefore, these results show that the α4nAChR subunit has a major role in liver damage. Similarly, Ehrlich et al. reported that the processes of bile duct ligation (BDL)-induced liver fibrosis were significantly attenuated in α7 KO mice [21]. This study also indicated the role of α7nAChR in the processes of liver injury. Contrary to these results, Kimura and colleagues found that the processes of hepatic inflammation and fibrosis were exacerbated in α7 KO mice [22]. Therefore, protective roles for α7nAChR have been identified in some studies. The protective role of α7nAChR is exerted through the cholinergic anti-inflammatory pathway in the liver [23]. Considering these contradictory results, in the current study, our main goal was to investigate the protective or detrimental effects of nicotine exposure on the processes of liver fibrosis in an experimental animal model. Nicotine may exert its effects on the progression of liver fibrosis by changing the expression of nAChRs. Considering that the expressional changes of these receptors during liver fibrosis and the effect of nicotine on their expression have not been determined yet, this study aims to explore the effect of nicotine exposure on the expression of nAChRs and α-smooth muscle actin (α-SMA; liver fibrosis marker). It is hoped that by clarifying the effect of nicotine and its relationship with the progression of liver fibrosis, it will be possible to introduce new therapeutic targets for the management of liver fibrosis.
Material and methods
Animals and ethics statement
In this experimental study, 36 male Wistar rats weighing ~220 g (eight weeks old) were purchased from the animal house of the Faculty of Medicine, Tabriz University of Medical Sciences. A week was given to the rats to adapt to the animal environment of the house. All animal maintenance and procedures were under recommendations established by the Animal Ethics Committee of Tabriz University of Medical Sciences and the protocol was approved (Ethical code: IR.TBZMED.AEC.1401.066). The BDL surgery was conducted under deep anesthesia, and all efforts were made to reduce the pain and suffering of the rats [24]. In this study, BDL was used as an animal model of liver fibrosis. Briefly, a midline abdominal incision was made under general anesthesia induced by intraperitoneal injection of ketamine and xylazine 100 and 10 mg/kg, respectively. The peritoneal cavity was opened and the common bile duct was isolated and triply ligated [25]. The sham-operated rats underwent a similar operation but without ligation of the bile duct.
Experimental plan
The rats were randomly divided into six groups: Sham + Saline, Sham + Nicotine (1 mg/kg), Sham + Nicotine (10 mg/kg), BDL + Saline, BDL + Nicotine (1 mg/kg), and BDL + Nicotine (10 mg/kg). Nicotine was purchased from Sigma-Aldrich (product number N3876) and the animals were treated intraperitoneally with or without nicotine injection every other day for 3 weeks [26, 27]. Each cigarette has approximately 1.5 mg of nicotine [28]. Thus, a pack of 20 cigarettes has approximately 30 mg of nicotine. If we convert the human dose of nicotine to a rat dose, we get approximately 10 mg/kg every other day [29]. Therefore, the doses we chose for low and high doses of nicotine (1 or 10 mg/kg) are equivalent to the concentration of nicotine found in light smokers and heavy smokers, respectively [30]. Some previous studies on normal rats or using various animal models of liver disease emphasize that nicotine may aggravate the process of liver fibrosis [31–33]. Because of this evidence, we expected to observe aggravation of fibrotic processes after chronic administration of nicotine in BDL rats. Therefore, we chose a model with the 3-week BDL which exhibits submaximal liver fibrosis and can investigate either protection or exaggeration of hepatic fibrosis in nicotine-treated rats [34]. After three weeks, the rats were sacrificed using an anesthetic overdose to measure the weight of the livers and spleens and take samples of the liver tissue for histological and molecular studies.
Liver/body and spleen/body weight ratio
After 3 weeks of BDL, both cholestatic and control animals were weighed and sacrificed and the livers and spleens were weighed using an analytical balance and the results were expressed in grams (g) to calculate the liver/body and spleen/body weight ratio. Portal hypertension, described as raised pressure in the portal vein, develops due to enhanced intrahepatic vascular resistance, frequently arising from liver fibrosis. Previous studies indicated that there was a high correlation between portal pressure and the spleen/body ratio [35, 36]. Therefore, in this study, the spleen/body ratio was measured as a measure of portal vein pressure.
Hematoxylin-eosin (H&E) and Masson trichrome staining and scoring
The liver tissues were fixed with 10% formaldehyde and the paraffin-embedded samples were sliced into 5 µm-thick sections. Histological assessment of fibrosis and ductular proliferation were evaluated using H&E as well as Masson trichrome staining. Generally, Masson’s trichrome staining is used for the detection of collagen fibers in tissues such as the liver, heart, skin, etc. on formalin-fixed, paraffin-embedded sections. In this technique, the collagen fibers will be stained blue and the nuclei will be stained black and the background is stained red. In our study, the liver fibrosis was staged 0-4 based on Scheuer’s scoring system (0 – no fibrosis, 1 – expansion of portal tract without linkage, 2 – portal expansion with the portal to portal linkage; 3 – an extensive portal to portal and focal portal to central linkage, 4 – cirrhosis) [37]. Ductular proliferation was graded on a scale of 0-3, with 0 = absent, 1 = mild, 2 = moderate, and 3 = severe [25].
RNA extraction and quantitative reverse transcription PCR
The relative mRNA levels were assessed by quantitative reverse transcription PCR (qRT-PCR) [38]. After Trizol (Invitrogen, Carlsbad, CA) treatment, all RNAs of the liver tissues were obtained and the Prime Script Kit (TaKaRa Bio Inc., Japan) was performed for cDNA synthesis. qRT-PCR was performed on a ViiATM7 RT-PCR system (Applied Biosystems, Carlsbad, CA) using SYBR Green fluorescent-based assay (638320, TaKaRa Bio Inc. Japan). The β-actin gene was used as an internal standard and after normalizing to its expression level, the relative expression levels of α4nAChR, α7nAChR, and α-SMA were quantified by the 2−ΔΔCt method [39]. The reaction parameters were set as 50°C for 2 min, 95°C for 15 s, 95°C for 15 s, and 60°C for 1 min for 40 cycles. The primers of target genes were synthesized by Sinaclon (Tehran, Iran) and reported in Table 1.
Table 1
Sequences of primers for α4nAChR, α7nAChR, αSMA, and β-actin genes
Statistical analyses
The data in the present study were expressed as mean ±SEM, and were analyzed using one-way ANOVA followed by Bonferroni post-test analyses for the test of differences between groups. Two-way ANOVA was used when the effect of the two variables was assessed. All statistical analyses were performed using GraphPad Prism 6 software (GraphPad Software, La Jolla California, USA). P-values < 0.05 were considered significant.
Results
Effect of nicotine administration on the liver/body and spleen/body weight ratios
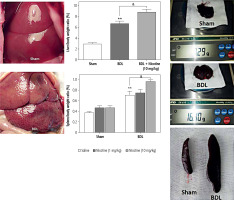
Rats were injected with low and high doses of nicotine for 21 days, and the effects of nicotine on their body weight and liver/spleen weight were evaluated (Fig. 1). Over the course of the study, there were no significant changes in the amount of feed consumed (vs. control values) by rats in the nicotine-treated groups. The gross anatomy of the liver shows the change of the liver from a smooth and polished state to a granular and enlarged state (Fig. 1). The results showed that the body weight of the BDL rats was significantly lower compared to the sham groups. In contrast, the liver and spleen weights in BDL rats were significantly higher compared to the control values at day 21. Thus, the liver/body and spleen/body weight ratio in BDL groups were significantly higher than the control values (p < 0.01). With the injection of a high dose of nicotine (10 mg/kg) in the BDL groups, the liver/body and the spleen/body weight ratio increased significantly (p < 0.05), while there was no difference in the values related to the low dose of nicotine (1 mg/kg) (Fig. 1). The gross morphology of the liver showed that high doses of nicotine led to an increase in extracellular matrix deposition in the liver and an increase in portal pressure and subsequently an increase in liver/body and spleen/body weight ratio, respectively. Similar to the ineffectiveness of low doses of nicotine on the liver/body and spleen/body weight ratio, we did not observe any effect of low doses of nicotine in other histological and molecular studies (data not shown). Considering that the administration of nicotine in high doses causes an increase in the weight of the spleens, it can be predicted that the high consumption of cigarettes in people with liver diseases leads to an increase in the complications of the disease caused by an increase in portal vein pressure.
Fig. 1
Gross morphology of sham and BDL-operated livers and spleens and liver/body and spleen/body weight ratios. The results showed that liver/body and spleen/body weight ratios were significantly higher in BDL rats vs. the sham-operated rats at day 21. **p < 0.01 in comparison with the sham group. High doses of nicotine led to an increase in portal pressure and subsequently an increase in liver/body and spleen/body weight ratios. &p < 0.05 in comparison with the BDL-saline group. Data are shown as mean ±SEM
Effect of nicotine exposure on ductular proliferation and liver fibrosis
The histology of liver fibrosis was studied using H&E and Masson’s trichrome staining (Table 2). BDL was associated with bile duct proliferation (Fig. 2) and extensive bridging fibrosis (portal to portal and portal to central linkage with fibrotic bands) (Fig. 3). As shown in these figures, nicotine exerted a significant effect on hepatic fibrosis and portal expansion in BDL rats. Likewise, nicotine in sham-operated rats altered the histological structure of the liver as assessed by H&E and trichrome staining.
Fig. 2
Representative photomicrographs of H&E-stained livers. The histological structure of control rat tissues was normal and ductular proliferation was absent. The livers of nicotine-treated rats had some degree of portal tract expansion. Portal expansion with the portal-to-portal linkage was the distinctive feature of the BDL group. Livers of nicotine-treated BDL rats displayed different degrees of pathological damage
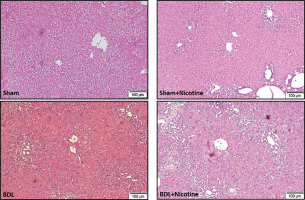
Fig. 3
Hepatic fibrosis was assessed in sham-operated and BDL rats with and without nicotine. Three weeks after the surgical procedure, liver tissues were obtained and collagen fibers were stained with Masson’s trichrome. BDL was associated with extensive bridging fibrosis (portal to portal and portal to central linkage with fibrotic bands). The deposition of fibrotic tissue was higher in the rats of the BDL group that received nicotine injections
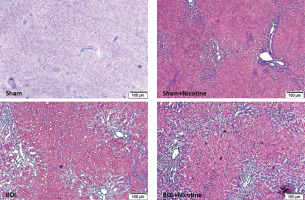
Table 2
Histological assessment of fibrosis and ductular proliferation of the liver
Quantitative RT-PCR showed that nicotine altered the gene expression
In the present study, we looked at the changes in the expression of α4nAChR and α7nAChR genes following the development of liver fibrosis induced by BDL as well as treatment with nicotine and its relationship with α-SMA gene expression (as a marker of liver fibrosis). The obtained results showed that the process of liver fibrosis with BDL alone led to an increase in the expression of α4nAChR (Fig. 4), α7nAChR (Fig. 5), and α-SMA (Fig. 6) genes. Also, nicotine alone (10 mg/kg) led to an increase in gene expression. Low doses of nicotine did not have statistically significant effects on gene expression (data not shown). However, high doses of nicotine augmented the target gene’s expression following nicotine treatment, and the expression level of target genes in BDL groups increased more with nicotine administration. It seems that α4nAChRs and α7nAChRs upregulation is associated with fibrosis in BDL rats.
Fig. 4
Quantitative analysis of hepatic mRNA levels of α4nAChR in shamoperated and BDL rats. α4nAChR mRNA expression was assessed using realtime RT-PCR and was expressed in relation to β-actin as an internal standard. BDL alone increased expression of α4nAChR. *p < 0.05 in comparison with the sham + saline group. Nicotine (10 mg/kg) led to an increase in α4nAChR expression. &p < 0.05 in comparison with the BDL-saline group. Data are shown as mean ±SEM
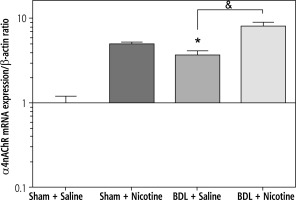
Fig. 5
The mRNA expression of α7nAChR was assessed by quantitative real-time RT-PCR and was expressed in relation to β-actin as an internal standard. In the group of control rats, nicotine (10 mg/kg) alone caused a significant increase in expression of α7nAChR. ****p < 0.0001 in comparison with the sham + saline group. Similarly, in the group of BDL rats, nicotine caused an increase in the expression of α7nAChR. &p < 0.05 in comparison with the BDL-saline group. Data are shown as mean ±SEM
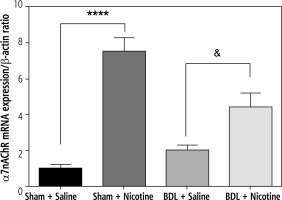
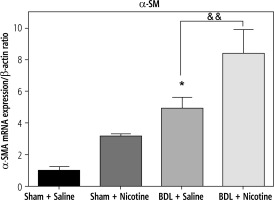
Fig. 6
Expression of α-SMA in rat livers obtained from sham-operated and BDL rats in relation to β-actin as an internal standard. BDL increased this marker of liver fibrosis. *p < 0.05 in comparison with the sham + saline group. Nicotine administration caused a further increase in its expression. &&p < 0.01 in comparison with the BDL-saline group. Data are shown as mean ±SEM
Discussion
The adverse effects of cigarette smoking on the lungs and cardiovascular system are well described; however, the detrimental effects of smoking on the liver are not as well defined [40, 41]. Therefore, the data in this regard are limited, and more research is needed to better understand how smoking and nicotine affect the liver. In a previous study, we examined the dose-dependent effects of nicotine on liver cell lines [38]. In the present study, our aim was to investigate the effects of nicotine in an animal model of liver fibrosis. Liver fibrosis is caused by various factors that damage the liver tissue [42]. This disease, which involves excessive accumulation of extracellular matrix proteins including collagen, can occur in most types of chronic liver diseases. Liver fibrosis is a response generated as a result of chronic liver injury due to various factors, such as alcohol consumption, non-alcoholic steatohepatitis, viral hepatitis B, and hepatitis C, autoimmune hepatitis, non-alcoholic fatty liver disease, and cholestatic liver diseases [7]. The elevated risk of liver fibrosis is exacerbated by smoking [43].
Cholestasis occurs due to the failure of bile secretions to enter the small intestine. If left untreated, cholestasis can cause serious damage to various organs of the body. Also, this disorder can affect the expression of various genes. In the present study, an increase in the expression of both subtypes of nAChRs was observed following the administration of nicotine. Also, the expression of these genes increases after BDL-induced liver damage. Nicotine administration led to a further increase in the expression of both types of receptors. Therefore, it seems that the fibrogenic effects of nicotine are largely mediated by increasing the gene expression of nicotinic receptors. In our previous study, using the same liver fibrosis model, we obtained similar results [25]. Inhibiting the activity of nicotinic receptors by using selective hepatic vagotomy reduced liver damage and the level of liver enzymes [25]. The results obtained from this study are in line with previous studies and show that the higher the expression of nAChRs in the liver, the higher the level of liver damage and subsequent liver fibrosis. Therefore, to treat liver fibrosis, especially in smokers, targeting these receptors can be a successful therapeutic strategy. The importance of cholestasis has inspired much research to be conducted to investigate the causative factors in its pathogenesis. Various factors such as atresia or absence of the bile duct, use of some drugs, family history, infection, pregnancy and autoimmune diseases, and genetic and metabolic factors have been proposed as the parameters involved in the occurrence of cholestasis. Cholestasis may occur extrahepatically due to obstruction of the bile ducts, such as the presence of stones and tumors, or intrahepatically, due to disorders in the formation of bile by hepatocytes and bile duct cells. It can eventually lead to liver fibrosis and cirrhosis. In most cases of acute injuries, with the disappearance of the causative agent, the liver will find its normal function and structure and no trace of damage will remain [44]. It is very important to accurately estimate the amount of liver fibrosis in the evaluation of prognosis, surveillance, and the adoption of therapeutic measures in chronic liver disease. Progressive liver fibrosis is one of the symptoms of chronic liver damage, which can eventually lead to cirrhosis, liver failure, and hepatocellular carcinoma [45].
Nicotine affects the body through nAChRs. In vertebrates, 17 different subunits including α1-10, β1-4, δ, ε, γ have been identified for nicotinic receptors, which are grouped together as homopentamers or heteropentamers and form a large family of different types of receptors [46]. Subunits α4 and α7 also play a role in anti-inflammatory pathways and may be increased as protective factors during liver fibrosis [47, 48]. α7nAChR are a family of ligand-dependent ion channels, which are composed of a homopentamer of five α7 subunits. These receptors are expressed by the CHRNA7 gene located on chromosome 15q14, and its final product is a protein with a molecular weight of 55 kilodaltons [49]. These receptors are expressed in almost all cells of the body and play a variety of roles. There are many findings about the effects of nicotine through nicotinic receptors [38, 50]. The intracellular signaling pathways of these receptors have been studied [31, 51-53]. Various studies have shown that smoking may cause liver fibrosis, but the possible mechanisms involved are unclear [51]. So far, no study has investigated the effects of nicotine administration on the development of liver fibrosis using the common bile duct ligation method. The results of this study have shown that nicotine, as a main compound in cigarette smoke, acts through α4nAChR and α7nAChR in the processes of liver fibrosis.
The current study was conducted to investigate the effect of nicotine administration on the gene expression of α4nAChR and α7nAChR in an animal model of liver fibrosis. For this purpose, first, the method of closing the bile duct (BDL) was used in male Wistar rats to create a model of liver fibrosis. Then, nicotine was applied in a low or high dose (1 or 10 mg/kg). Chronic nicotine exposure upregulated nicotinic receptors [54]. The fibrosis model was the main topic of our study and we investigated liver fibrosis through Masson’s trichrome staining and αSMA gene expression analysis. In addition to these markers, we also checked the liver/body weight ratio, but in-depth analysis of other mRNA or protein expression for various fibrosis-related factors and additional pharmacodynamic experiments with other available well-known agonists and antagonists of nAChR are needed to corroborate the data. Also, because nicotine binds to their receptors (nAChRs), treatment of nicotine may affect the mRNA expression of nAChRs. Therefore, we also measured the expression of nAChRs. Further studies using receptor inhibitors are essential to reveal a mechanistic link between nAChR expression and the severity of fibrosis in rats. Based on previous studies, nicotine is a fibrogenic compound [51]. However, until now, the mechanisms of nAChRs in liver fibrosis and the effect of nicotine on the expression level of these receptors in liver fibrosis remain unclear. The current study showed the changes in the expression of α4nAChR and α7nAChR following treatment with nicotine and their relationship with the liver fibrosis indexes.
Conclusions
Liver fibrosis is one of the most common human diseases, and every year it causes the death of thousands of people. Considering the high prevalence of liver fibrosis and the lack of definitive and appropriate treatment, it is crucial to investigate and understand the pathogenesis of this disease from a molecular and cellular point of view to find new predictive and treatment methods and develop targeted therapeutic agents. In the current study, we evaluated the effect of nicotine administration on a BDL-induced liver fibrosis model. The changes in mRNA expression of several genes (α4nAchR, α7nAchR, α-SMA) were investigated together with histologic changes (H&E, and Masson’s trichrome staining) of liver tissues by nicotine treatment in bile duct-ligated rats. Such rats exhibit variation in α4nAChR and α7nAChR, and these receptors may mediate the effects of nicotine in the liver and may be a therapeutic target in managing extrahepatic biliary obstruction. Additional experiments that prove the causal link between them are required. According to the observed effects of nicotine in the context of liver fibrosis, it can be expected that smoking in people with liver fibrosis will lead to an increase in the complications of the disease. Considering the prevalence of the use of electronic cigarettes that contain high concentrations of nicotine, it should be taken seriously.






