Introduction
Venous thromboembolism (VTE), clinically manifested as deep vein thrombosis (DVT) or acute pulmonary embolism (PE), is the third most common acute cardiovascular syndrome following myocardial infarction and stroke [1]. The annual incidence of PE is between 39 and 115 per 100,000 inhabitants. The incidence of VTE is almost eight times higher in people aged 80 and older than in the fifth decade of life [2]. Therefore, with the incre- asing age of the population, long-term studies show an increasing tendency in the annual incidence rates of PE over time [3-6]. A recent analysis from the World Health Organization (WHO) mortality database (2000-2015) found an average of 38,929 PE-related deaths each year in 41 countries of the WHO European Region (including Central Asia) representing a population of approximately 651 million [7]. Between 2000 and 2015, the annual age-standardized death rates associated with PE decreased by almost 50% (from 12.7 to 6.5 deaths per 100,000 inhabitants) with no significant gender differences. Despite this overall positive trend, the study also found that PE-related mortality continues to increase exponentially with age, reaching or even exceeding 80 deaths per 100,000 elderly population, and that PE also remains a relatively important cause of (in comparison with other causes) deaths among younger women, in whom it accounted for up to 13 cases per 1000 deaths [7, 8].
COVID-19 first occurred in Wuhan (Hubei province) and the cause of the infection was soon identified as a coronavirus [1]. However, a great number of cases occurred without any in the Huanan animal market, which suggested human-to-human transmission. Since the outbreak of coronavirus disease 2019 (COVID-19), clinicians have struggled with the attempt to diagnose and manage the severe and fatal complications of COVID-19 appropriately. Several reports have described significant procoagulatory events, including life-threatening pulmonary embolism in these patients [9-58]. SARS-CoV-2 invades the host cell by using the superficial S protein complex (spike protein complex). The complex consists of two subunits: S1 and S2. The S1 domain binds the appropriate receptor on the host cell surface, and, at the same time, the S2 domain is responsible for membrane fusion [10]. Several studies have pointed to the ACE2 host cell receptor as a crucial molecule in SARS-CoV-2 invasion.
While ACE2 mRNA is found in all organs, ACE2 protein expression is detected in the heart, kidney, testis, lung type I and type II alveolar epithelial cells, nasal, and oral mucosa and nasopharynx (basal layer of the non- keratinizing squamous epithelium). Additional tissues include smooth muscle cells and endothelium of vessels from the stomach, small intestine and colon, in smooth muscle cells of the muscularis mucosae and the muscularis propria, in enterocytes of all parts of the small intestine including the duodenum, jejunum, and ileum (but not the colon), skin (basal cell layer of the epidermis extending to the basal cell layer of hair follicles, smooth muscle cells surrounding the sebaceous glands, cells of the eccrine glands), endothelial, and smooth muscle cells of the brain.
Affinity to the infected tissue shown by coronaviruses (CoVs) is dependent on surface cell ACE-2 expression. Recent publications suggest stronger affinity to ACE-2 presented by SARS-CoV-2 than to other known CoVs [11-13, 47-49]. The activity of S1 and S2 domains is controlled with the assistance of many enzymes such as cathepsin L, furin and transmembrane protease serine 2 (TMPRSS2). All of these enzymes are present in various tissues of the intestine, liver and lungs. It proves that SARS-CoV-2 could invade different systems and organs. A two-step model of cleavage was proposed: first separating S1 and S2, second activating the S2 site. S proteins could be cleaved by one of the specific proteases mentioned above. Co-expression of TMPRSS2 and ACE2 was found in pneumocytes, indicating the importance of TMPRSS2 in SARS-CoV-2 invasion. TMPRSS2 as a transmembrane enzyme cleaves the S1/S2 complex and activates the S2 domain. TMPRSS2 acts in the lumen of the viral endocytic vesicle. Interestingly, TMPRSS2 cleaves S proteins in many sites, creating molecules of 85, 110, 150, 45 and 55 kDa. Cleavage controlled by TMPRSS2 corresponded to SARS-CoV-2 infectivity [21, 45-48].
Material and methods
We performed a retrospective study on a group of 226 COVID-19 patients and selected group of patients who experienced an episode of pulmonary embolism. The analyzed group consisted of 136 men and 90 women with a mean age of 70 years (26-83). The two groups of patients were divided based on the percentage of the involved pulmonary tissue. Analyzed patients were hospitalized in Provincial Specialist Hospital Cardinal Stefan Wyszyński in Lublin.
All components were classified in accordance with the applicable regulations on sensitive data. Several selected inflammatory markers such as C-reactive protein, D-dimer, lymphocyte count, platelet count, as well as novel inflammatory biomarkers such as neutrophil to lymphocyte ratio and platelet to lymphocyte ratio, were analyzed. The neutrophil to lymphocyte ratio is calculated by dividing the absolute count for neutrophils by the absolute count for lymphocytes, while the platelet to lymphocyte ratio is calculated by platelet count divided by absolute lymphocyte count.
Each patient underwent high resolution computed tomography. The images were assessed by an experienced radiologist.
The study achieved full approval of the Medical University of Lublin Ethics Committee. Every single stage of the performed study was carried out in compliance with the Helsinki Declaration and national legislation.
Results
Two groups were distinguished among analyzed patients. The first group consisted of patients with < 50% of lung capacity changes seen in high resolution tomography scan, which covered 167 patients. The second group, with long capacity changes over > 50%, consisted of 79 patients. Among those there were 136 men (60%) and 90 women (39%) with the mean age of 70 years.
Previously mentioned inflammatory parameters were measured. A detailed descriptive analysis is presented in Tables 1-3.
Table 1
Combined presentation of gathered data. N is the number of performed analyses of selected parameters. Some of the parameters were measured more than once
Table 2
Presentation of gathered data in patients with < 50% of lung capacity changes. N is the number of performed analyses of selected parameters. Some of the parameters were measured more than once
Table 3
Presentation of gathered data in patients with < 50% of lung capacity changes. N is the number of performed analyses of selected parameters. Some of the parameters were measured more than once
Presented data were analyzed using the Spearman correlation coefficient, which revealed a statistically significant result regarding analyzed parameters, as presented in Table 4 (p < 0.05). A positive correlation was observed for C-reactive protein, neutrophil levels as well as neutrophil to lymphocyte ratio and platelet to lymphocyte ratio. A negative correlation was observed for lymphocyte count. Interestingly, there was no correlation for D-dimer, platelet count or interleukin 6 (IL-6) level.
Table 4
Spearman correlation coefficient analysis results
In this study the analysis of both groups was performed, using the Mann-Whitney U-test, which showed statistically significant results between groups. The group that consisted of patients with < 50% of lung capacity changes had higher parameter values for each analyzed parameter. Especially, the statistical significance of novel inflammatory markers was much higher than standard inflammatory markers such as C-reactive protein and IL-6 (Table 5).
Table 5
Mann-Whitney U-test statistical analysis
Discussion
The incidence of PE in hospitalized COVID-19 patients is approximately 1.9-8.9% [36, 40, 50, 51]. The retrospective nature of the analyzed cohorts and relatively short observation periods could have underestimated the actual incidence of PE. Critical COVID-19 patients requiring admission to the ICU seem to be at higher risk of thromboembolic complications, especially PE, which may occur in up to 26.6% of these patients [43]. In a prospective observational study involving 150 patients admitted to four ICU wards in two French hospitals, despite antithrombotic prophylaxis, the occurrence of PE was observed in 16.7% of treated patients [38]. The authors also reported that thromboembolic events occurred more frequently in patients with acute respiratory distress syndrome (ARDS) in COVID-19 patients compared to the historic ARDS cohort of a different etiology, underlining the unique procoagulatory effect of COVID-19 compared to other etiologies of ARDS. In a retrospective cohort of 184 COVID-19 patients admitted to the ICU in three hospitals in the Netherlands, it was found that 13.6% of patients developed PE despite anticoagulation [39]. Interestingly, the incidence of PE increased to 33.3% when the follow-up period was extended from 1 to 2 weeks [46], at a time when increased awareness of the frequent occurrence of PE could lead to a higher rate of suspicion and extended diagnosis to detect this complication. Similarly, Poissy et al. reported that 20.6% of patients admitted to a French ICU had pulmonary embolism on average 6 days after admission to the ICU despite the use of anticoagulants [41]. These authors also found that the incidence of PE in COVID-19 patients was twice as high as in patients admitted to the ICU as a control group and in 40 patients admitted to the ICU due to severe influenza. Abnormalities in various coagulation parameters have been frequently reported [49, 50] and were associated with poor prognosis [51].
Platelet to lymphocyte ratio (PLR), a novel marker of inflammation, was identified in several studies as an increased inflammatory response marker, suggesting worse prognosis and higher intensity of cytokine storm [59] (Fig. 1).
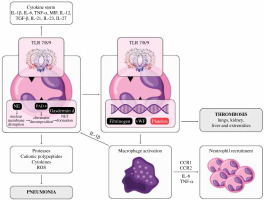
Fig. 1
The role of neutrophils and platelets in the cytokine storm during COVID-19 infection. The activation of neutrophils induces selected cytokine production, which contributes to the cytokine storm and thus results in ARDS. Interaction of SARS-CoV-2 with TLR 7/8/9 results in the formation of neutrophil extracellular traps (NET), which are web-like chromatin structures produced by neutrophils. The interaction of fibrin and platelets with NET can cause thrombosis in several organs, especially the lungs
A meta-analysis performed by Simadibrata et al. showed that a high PLR value was associated with severe COVID-19 [60]. Chung et al. claim that increased platelet activation occurs in acute PE, a higher RV/LV ratio reflecting a larger PE burden that may correspond to increased platelet consumption and therefore a smaller PLR [61]. This statement was confirmed by Phan et al., who found that PLR was significantly lower in patients with massive PE compared to patients with low-risk PE [62]. In this study platelet-to-lymphocyte ratio (PLR) was much higher in the group with < 50% of lung capacity changes in HR CT, which would be consistent with the hypothesis of Phan et al. Moreover, they also analyzed neutrophil-to-lymphocyte ratio and found out that a decrease in lymphocyte count coupled with minimal change in neutrophil and platelet counts influenced the increase in neutrophil-to-lymphocyte ratio (NLR) and PLR in nonsurvivors and the increased NLR and PLR were correlated with proinflammatory state. This study shows a similar positive correlation of NLR and negative correlation of lymphocyte count compared to the level of lung tissue involvement in HR CT.
Interleukin 6 is a soluble pro-inflammatory and immunoregulatory cytokine that contributes to host defense by stimulating acute phase responses, hematopoiesis and immune responses. Previous studies have shown significantly increased expression of IL-6 in both DVT patients and animal models. Moreover, blockade of IL-6 in a mouse study with the use of a specific antibody inhibited the formation and development of thrombosis [54-58]. In addition, the diagnosis of pulmonary embolism uses the level of D-dimers. In a study by Kara et al., compared to patients who did not have pulmonary embolism, patients with pulmonary embolism had a significantly higher mean level of D-dimers (pulmonary embolism, 6 ±7 µg/ml; no pulmonary embolism, 1 ±1 µg/ml, p = 0.001) [63]. Although statistically significant, this study shows that common inflammatory and thrombotic markers such as D-dimer level and IL-6 level have rather weak significance compared to novel inflammatory biomarkers when analyzing pulmonary embolism in COVID-19.
Conclusions
This study underlines the role of novel inflammatory biomarkers such as neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in patients with pulmonary embolism in COVID-19. We suggest that these biomarkers may have higher assessment value compared to routinely used biomarkers.
Limitations
There are some limitations of this study that have to be acknowledged. Large population studies should be carried in order to assess the importance and clinical usefulness of selected biomarkers. In this study a clinical control group would enhance the usefulness of selected biomarkers in COVID-19. In addition, for all the patients recruited, the registration of chronic conditions was not possible, due to the heterogeneity of data collections in the different wards.


